Ceramide lipid-based nanosuspension for enhanced delivery of docetaxel with synergistic antitumor efficiency
- PMID: 28502199
- PMCID: PMC8241063
- DOI: 10.1080/10717544.2016.1225853
Ceramide lipid-based nanosuspension for enhanced delivery of docetaxel with synergistic antitumor efficiency
Abstract
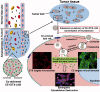
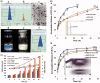
Ceramide (CE), a bioactive lipid with tumor suppression, has been widely used as a drug carrier and enhancer for cancer therapy. CE-based combination therapy was prone to be attractive in cancer therapy. In our previous study, the combination of CE and docetaxel (DTX) was proved to be an effective strategy for cancer therapy. To further improve the antitumor efficiency of DTX, the CE lipid-based nanosuspensions (LNS) was prepared for the delivery of DTX to exhibit synergistic therapeutic effect. The enhanced delivery and synergistic therapeutic effect of DTX-loaded CE-LNS (CE + DTX-LNS) were evaluated. CE + DTX-LNS exhibited spherical or ellipsoidal shape, uniform particle size distribution (108.1 ± 3.8 nm), sustained release characteristics and good stability in vitro. Notably, CE + DTX-LNS could effectively co-localize CE and DTX into same tumor cell and subsequently play synergistic cell damage effect compared with CE-LNS + DTX-LNS (p < 0.05). The in vivo fluorescence imaging results showed that CE + DTX-LNS could effectively prolong the in vivo circulation time and enhance the accumulation in tumor sites. Moreover, the antitumor efficacy of CE + DTX-LNS observed in B16 murine melanoma model was 93.94 ± 2.77%, significantly higher than that of CE-LNS, DTX-LNS, Duopafei® (p < 0.01) and CE-LNS + DTX-LNS (p < 0.05), respectively, demonstrating that co-delivery of CE and DTX into same tumor cell was the basis for enhanced synergistic therapeutic effect. Furthermore, histological examination of Blank-LNS showed no visible tissue toxicity compared to normal saline. Consequently, CE-LNS could effectively delivery DTX and CE + DTX-LNS exhibit synergistic inhibition of tumor growth due to the co-localization of CE and DTX. CE-LNS hold great potential to be an appropriate carrier for CE-based combination chemotherapy.
Keywords: Ceramide; co-delivery; combination therapy; docetaxel; lipid-based nanosuspension.
Conflict of interest statement
The authors confirm that this article content has no conflict of interest. The project was funded by the National Natural Science Foundation of China (No. 81573368) and the Science and Technology Development Project of Shandong Province (2014GGE27121).
Figures




Similar articles
-
Functionalized docetaxel-loaded lipid-based-nanosuspensions to enhance antitumor efficacy in vivo.Int J Nanomedicine. 2019 Apr 11;14:2543-2555. doi: 10.2147/IJN.S191341. eCollection 2019. Int J Nanomedicine. 2019. PMID: 31114190 Free PMC article.
-
Docetaxel-loaded-lipid-based-nanosuspensions (DTX-LNS): preparation, pharmacokinetics, tissue distribution and antitumor activity.Int J Pharm. 2011 Jul 15;413(1-2):194-201. doi: 10.1016/j.ijpharm.2011.04.023. Epub 2011 Apr 21. Int J Pharm. 2011. PMID: 21540085
-
Synergistic enhancement of cancer therapy using a combination of ceramide and docetaxel.Int J Mol Sci. 2014 Mar 10;15(3):4201-20. doi: 10.3390/ijms15034201. Int J Mol Sci. 2014. PMID: 24619193 Free PMC article.
-
Local administration of large surface area microparticle docetaxel to solid carcinomas induces direct cytotoxicity and immune-mediated tumoricidal effects: preclinical and clinical studies.Drug Deliv Transl Res. 2023 Feb;13(2):503-519. doi: 10.1007/s13346-022-01226-2. Epub 2022 Sep 4. Drug Deliv Transl Res. 2023. PMID: 36058988 Free PMC article. Review.
-
Nanoparticles Loaded with Docetaxel and Resveratrol as an Advanced Tool for Cancer Therapy.Biomedicines. 2022 May 20;10(5):1187. doi: 10.3390/biomedicines10051187. Biomedicines. 2022. PMID: 35625921 Free PMC article. Review.
Cited by
-
Application of sphingolipid-based nanocarriers in drug delivery: an overview.Ther Deliv. 2024;15(8):619-637. doi: 10.1080/20415990.2024.2377066. Epub 2024 Jul 29. Ther Deliv. 2024. PMID: 39072358 Free PMC article. Review.
-
Functionalized docetaxel-loaded lipid-based-nanosuspensions to enhance antitumor efficacy in vivo.Int J Nanomedicine. 2019 Apr 11;14:2543-2555. doi: 10.2147/IJN.S191341. eCollection 2019. Int J Nanomedicine. 2019. PMID: 31114190 Free PMC article.
-
Sphingolipid metabolism and drug resistance in ovarian cancer.Cancer Drug Resist. 2018;1:181-197. doi: 10.20517/cdr.2018.06. Epub 2018 Sep 19. Cancer Drug Resist. 2018. PMID: 31891125 Free PMC article.
-
A localized hydrogel-mediated chemotherapy causes immunogenic cell death via activation of ceramide-mediated unfolded protein response.Sci Adv. 2023 Jun 30;9(26):eadf2746. doi: 10.1126/sciadv.adf2746. Epub 2023 Jun 30. Sci Adv. 2023. PMID: 37390205 Free PMC article.
-
Self-delivering prodrug-nanoassemblies fabricated by disulfide bond bridged oleate prodrug of docetaxel for breast cancer therapy.Drug Deliv. 2017 Nov;24(1):1460-1469. doi: 10.1080/10717544.2017.1381201. Drug Deliv. 2017. PMID: 28950729 Free PMC article.
References
-
- Adkins SS, Hobbs HR, Benaissi K, et al. . (2008). Stable colloidal dispersions of a lipase-perfluoropolyether complex in liquid and supercritical carbon dioxide. J Phys Chem B 112:4760–9 - PubMed
-
- Chi Le NU, Tabuchi K, Nakamagoe M, et al. . (2015). Ceramide/sphingomyelin cycle involvement in gentamicin-induced cochlear hair cell death. Arch Toxicol 89:415–21 - PubMed
-
- Cho HJ, Yoon HY, Koo H, et al. . (2011). Self-assembled nanoparticles based on hyaluronic acid-ceramide (HA-CE) and Pluronic® for tumor-targeted delivery of docetaxel. Biomaterials 32:7181–90 - PubMed
-
- Choudhary GS, Al-Harbi S, Almasan A. (2015). Caspase-3 activation is a critical determinant of genotoxic stress-induced apoptosis. Methods Mol Biol 1219:1–9 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
