Phase II trial of the PI3 kinase inhibitor buparlisib (BKM-120) with or without enzalutamide in men with metastatic castration resistant prostate cancer
- PMID: 28502694
- PMCID: PMC6345641
- DOI: 10.1016/j.ejca.2017.02.030
Phase II trial of the PI3 kinase inhibitor buparlisib (BKM-120) with or without enzalutamide in men with metastatic castration resistant prostate cancer
Abstract
Background: Phosphatidylinositol-3-kinase (PI3K) and androgen receptor pathway activation is common in metastatic castration resistant prostate cancer (mCRPC). Buparlisib is an oral, pan-class I PI3 kinase inhibitor.
Methods: This was a multisite single arm phase II trial of buparlisib 100 mg ± enzalutamide daily in men with mCRPC whose disease progressed on or who were not candidates for docetaxel. The primary end-point was the rate of radiographic/clinical progression-free survival (PFS) at 6 months.
Results: Thirty men were accrued: 67% post-docetaxel; median prostate specific antigen (PSA) was 70 ng/dl, 83% had ≥4 prior therapies for mCRPC; 43% received concurrent enzalutamide. The final 6 month PFS rate was estimated to be 10% (95% confidence interval 2.5-23.6%). Median PFS was 1.9 months and was 3.5 months with concurrent enzalutamide. Median overall survival was 10.6 months. Concurrent enzalutamide led to a five-fold reduction in buparlisib concentrations. PSA declines were observed in 23%; no patients achieved a ≥50% decline, and no radiographic responses were observed. Severe adverse events occurred in four men including respiratory infection and multi-organ failure, urinary tract obstruction, confusion and one seizure in the setting of a new central nervous system (CNS) metastasis. Grade III adverse events were seen in 43% of patients; common toxicities included grade I-II weight loss, diarrhoea, nausea, fatigue, anorexia, rash, hyperglycemia and anxiety/mood disorders.
Conclusions: Buparlisib did not demonstrate significant activity in men with mCRPC, suggesting that PI3K inhibition is not sufficient to reverse resistant mCRPC progression. Future studies of PI3K pathway inhibitors with concurrent enzalutamide should develop optimal dosing and focus on selected patients more likely to benefit.
Keywords: BKM-120; Buparlisib; Clinical trial; Enzalutamide; Metastatic castration resistant prostate cancer; PI3 kinase; Prostate cancer.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict of interest statement
The other authors report no potential conflicts of interest.
Figures
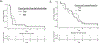
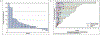
Comment in
-
Attaining precision therapy in prostate cancer: A tall order.Eur J Cancer. 2017 Aug;81:226-227. doi: 10.1016/j.ejca.2017.05.011. Epub 2017 Jun 16. Eur J Cancer. 2017. PMID: 28629596 Free PMC article. No abstract available.
References
-
- Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 2010;363:411–22. - PubMed
-
- de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, Kocak I, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet 2010; 376:1147–54. - PubMed
-
- Parker C, Nilsson S, Heinrich D, O’Sullivan JM, Fossa SD, Chodacki A, et al. Updated analysis of the phase III, doubleblind, randomized, multinational study of radium-223 chloride in castration-resistant prostate cancer (CRPC) patients with bone metastases (ALSYMPCA). ASCO Meeting Abstracts, vol. 30;
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

