Tracing the Enterococci from Paleozoic Origins to the Hospital
- PMID: 28502769
- PMCID: PMC5499534
- DOI: 10.1016/j.cell.2017.04.027
Tracing the Enterococci from Paleozoic Origins to the Hospital
Abstract
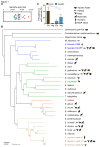
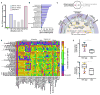
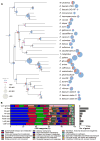
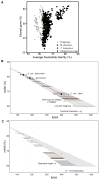
We examined the evolutionary history of leading multidrug resistant hospital pathogens, the enterococci, to their origin hundreds of millions of years ago. Our goal was to understand why, among the vast diversity of gut flora, enterococci are so well adapted to the modern hospital environment. Molecular clock estimation, together with analysis of their environmental distribution, phenotypic diversity, and concordance with host fossil records, place the origins of the enterococci around the time of animal terrestrialization, 425-500 mya. Speciation appears to parallel the diversification of hosts, including the rapid emergence of new enterococcal species following the End Permian Extinction. Major drivers of speciation include changing carbohydrate availability in the host gut. Life on land would have selected for the precise traits that now allow pathogenic enterococci to survive desiccation, starvation, and disinfection in the modern hospital, foreordaining their emergence as leading hospital pathogens.
Keywords: Enterococcus; Paleozoic; antibiotic resistance; commensal; early life; hospital acquired infection; origins; speciation; terrestrialization.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures


Comment in
-
Bacterial evolution: The origins of pathogenic enterococci.Nat Rev Microbiol. 2017 Jul;15(7):382-383. doi: 10.1038/nrmicro.2017.65. Epub 2017 Jun 5. Nat Rev Microbiol. 2017. PMID: 28579609 No abstract available.
References
-
- Aarestrup FM, Butaye P, Witte W. Nonhuman Reservoirs of Enterococci. In: Gilmore M, editor. The Enterococci. American Society of Microbiology; 2002. pp. 55–99.
-
- Adams Krumins J, van Oevelen D, Martijn Bezemer T, De Deyn GB, Gera Hol WH, van Donk E, de Boer W, de Ruiter PC, Middelburg JJ, Monroy F, et al. Soil and Freshwater and Marine Sediment Food Webs: Their Structure and Function. BioScience. 2013;63:35–42.
-
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. - PubMed
-
- Azam F, Malfatti F. Microbial structuring of marine ecosystems. Nat Rev Microbiol. 2007;5:782–91. Review. Erratum in: Nat Rev Microbiol. (2007). 5:966. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

