Checks and balances: The glucocorticoid receptor and NFĸB in good times and bad
- PMID: 28502781
- PMCID: PMC5523465
- DOI: 10.1016/j.yfrne.2017.05.001
Checks and balances: The glucocorticoid receptor and NFĸB in good times and bad
Abstract
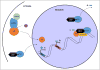
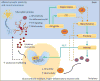
Mutual regulation and balance between the endocrine and immune systems facilitate an organism's stress response and are impaired following chronic stress or prolonged immune activation. Concurrent alterations in stress physiology and immunity are increasingly recognized as contributing factors to several stress-linked neuropsychiatric disorders including depression, anxiety, and post-traumatic stress disorder. Accumulating evidence suggests that impaired balance and crosstalk between the glucocorticoid receptor (GR) and nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) - effectors of the stress and immune axes, respectively - may play a key role in mediating the harmful effects of chronic stress on mood and behavior. Here, we first review the molecular mechanisms of GR and NFκB interactions in health, then describe potential shifts in the GR-NFκB dynamics in chronic stress conditions within the context of brain circuitry relevant to neuropsychiatric diseases. Furthermore, we discuss developmental influences and sex differences in the regulation of these two transcription factors.
Keywords: Anxiety; Depression; FKBP5; Glucocorticoid receptor; Glucocorticoid resistance; Inflammation; Nuclear factor kappa-light-chain-enhancer of activated B cells; PTSD; Stress.
Copyright © 2017 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors have no conflicting interests.
Figures



References
-
- Adcock IM, Barnes PJ. Molecular mechanisms of corticosteroid resistance. Chest. 2008;134(2):394–401. - PubMed
-
- Adzic M, Djordjevic J, Mitic M, Brkic Z, Lukic I, Radojcic M. The contribution of hypothalamic neuroendocrine, neuroplastic and neuroinflammatory processes to lipopolysaccharide-induced depressive-like behaviour in female and male rats: Involvement of glucocorticoid receptor and C/EBP-beta. Behav Brain Res. 2015;291:130–139. - PubMed
-
- Akiyama T, Shiraishi T, Qin J, Konno H, Akiyama N, Shinzawa M, Miyauchi M, Takizawa N, Yanai H, Ohashi H, Miyamoto-Sato E, Yanagawa H, Yong W, Shou W, Inoue J. Mitochondria-nucleus shuttling FK506-binding protein 51 interacts with TRAF proteins and facilitates the RIG-I-like receptor-mediated expression of type I IFN. PLoS One. 2014;9(5):e95992. - PMC - PubMed
-
- Ang E, Chen J, Zagouras P, Magna H, Holland J, Schaeffer E, Nestler EJ. Induction of nuclear factor-kappaB in nucleus accumbens by chronic cocaine administration. J Neurochem. 2001;79(1):221–224. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

