Branch Duct Intraductal Papillary Mucinous Neoplasms of the Pancreas Involving Type 1 Localized Autoimmune Pancreatitis with Normal Serum IgG4 Levels Successfully Diagnosed by Endoscopic Ultrasound-guided Fine-needle Aspiration and Treated without Pancreatic Surgery
- PMID: 28502930
- PMCID: PMC5491810
- DOI: 10.2169/internalmedicine.56.8017
Branch Duct Intraductal Papillary Mucinous Neoplasms of the Pancreas Involving Type 1 Localized Autoimmune Pancreatitis with Normal Serum IgG4 Levels Successfully Diagnosed by Endoscopic Ultrasound-guided Fine-needle Aspiration and Treated without Pancreatic Surgery
Abstract
We herein report a 68-year-old man with branch duct intraductal papillary mucinous neoplasms of the pancreas (BD-IPMNs) involving type 1 localized autoimmune pancreatitis (AIP) with normal serum IgG4 levels. Although he was referred to our medical center due to suspicion of pancreatic cancer concomitant with BD-IPMNs, endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) revealed a mass suspected of being pancreatic cancer to be type 1 AIP. Steroid administration notably reduced the mass. Although the clinical diagnosis of pancreatic masses in patients with IPMN can be occasionally challenging, performing a pathological examination by EUS-FNA may prevent unnecessary pancreatic surgery in cases of possible AIP.
Keywords: autoimmune pancreatitis (AIP); endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA); intraductal papillary mucinous neoplasms of the pancreas (IPMN).
Figures

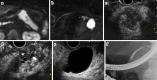

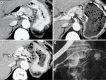
References
-
- Uehara H, Nakaizumi A, Ishikawa O, et al. . Development of ductal carcinoma of the pancreas during follow-up of branch duct intraductal papillary mucinous neoplasm of the pancreas. Gut 57: 1561-1565, 2008. - PubMed
-
- Tanno S, Nakano Y, Koizumi K, et al. . Pancreatic ductal adenocarcinomas in long-term follow-up patients with branch duct intraductal papillary mucinous neoplasms. Pancreas 39: 36-40, 2010. - PubMed
-
- Naitoh I, Nakazawa T, Notohara K, et al. . Intraductal papillary mucinous neoplasm associated with autoimmune pancreatitis. Pancreas 42: 552-554, 2013. - PubMed
-
- Shiokawa M, Kodama Y, Yoshimura K, et al. . Risk of cancer in patients with autoimmune pancreatitis. Am J Gastroenterol 108: 610-617, 2013. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

