Stress-Related and Circadian Secretion and Target Tissue Actions of Glucocorticoids: Impact on Health
- PMID: 28503165
- PMCID: PMC5408025
- DOI: 10.3389/fendo.2017.00070
Stress-Related and Circadian Secretion and Target Tissue Actions of Glucocorticoids: Impact on Health
Abstract
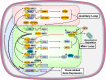
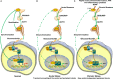
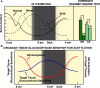
Living organisms are highly complex systems that must maintain a dynamic equilibrium or homeostasis that requires energy to be sustained. Stress is a state in which several extrinsic or intrinsic disturbing stimuli, the stressors, threaten, or are perceived as threatening, homeostasis. To achieve homeostasis against the stressors, organisms have developed a highly sophisticated system, the stress system, which provides neuroendocrine adaptive responses, to restore homeostasis. These responses must be appropriate in terms of size and/or duration; otherwise, they may sustain life but be associated with detrimental effects on numerous physiologic functions of the organism, leading to a state of disease-causing disturbed homeostasis or cacostasis. In addition to facing a broad spectrum of external and/or internal stressors, organisms are subject to recurring environmental changes associated with the rotation of the planet around itself and its revolution around the sun. To adjust their homeostasis and to synchronize their activities to day/night cycles, organisms have developed an evolutionarily conserved biologic system, the "clock" system, which influences several physiologic functions in a circadian fashion. Accumulating evidence suggests that the stress system is intimately related to the circadian clock system, with dysfunction of the former resulting in dysregulation of the latter and vice versa. In this review, we describe the functional components of the two systems, we discuss their multilevel interactions, and we present how excessive or prolonged activity of the stress system affects the circadian rhythm of glucocorticoid secretion and target tissue effects.
Keywords: circadian endocrine rhythms; clock system; glucocorticoid receptor; glucocorticoids; hypothalamic–pituitary–adrenal axis; stress; stress system.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

