Fluorinated cyclohexanes: Synthesis of amine building blocks of the all- cis 2,3,5,6-tetrafluorocyclohexylamine motif
- PMID: 28503208
- PMCID: PMC5405691
- DOI: 10.3762/bjoc.13.72
Fluorinated cyclohexanes: Synthesis of amine building blocks of the all- cis 2,3,5,6-tetrafluorocyclohexylamine motif
Abstract
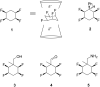
This paper reports the synthesis of three amine stereoisomers 5a-c of the tetrafluorocyclohexyl ring system, as building blocks for discovery chemistry programmes. The synthesis starts from a Birch reduction of benzonitrile, followed by an in situ methyl iodide quench. The resultant 2,5-cyclohexadiene was progressed via double epoxidations and then hydrofluorination ring opening reactions. The resultant fluorohydrin moieties were then converted to different stereoisomers of the tetrafluorocyclohexyl ring system, and then reductive hydrogenation of the nitrile delivered three amine stereoisomers. It proved necessary to place a methyl group on the cyclohexane ring in order to stabilise the compound against subsequent HF elimination. The two all-cis tetrafluorocyclohexyl isomers 5a and 5b constitute facially polarized cyclohexane rings, with fluorines on the electronegative face and hydrogens on the electropositive face.
Keywords: all-cis tetrafluorocyclohexane motif; deoxofluorination reactions; fluorinated amines; fluorinated cyclohexanes.
Figures
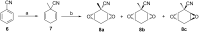


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
