Reconfigurable microfluidic device with discretized sidewall
- PMID: 28503247
- PMCID: PMC5415406
- DOI: 10.1063/1.4983148
Reconfigurable microfluidic device with discretized sidewall
Abstract
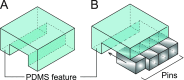
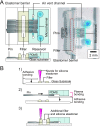
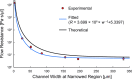
Various microfluidic features, such as traps, have been used to manipulate flows, cells, and other particles within microfluidic systems. However, these features often become undesirable in subsequent steps requiring different fluidic configurations. To meet the changing needs of various microfluidic configurations, we developed a reconfigurable microfluidic channel with movable sidewalls using mechanically discretized sidewalls of laterally aligned rectangular pins. The user can deform the channel sidewall at any time after fabrication by sliding the pins. We confirmed that the flow resistance of the straight microchannel could be reversibly adjusted in the range of 101-105 Pa s/μl by manually displacing one of the pins comprising the microchannel sidewall. The reconfigurable microchannel also made it possible to manipulate flows and cells by creating a segmented patterned culture of COS-7 cells and a coculture of human umbilical vein endothelial cells (HUVECs) and human lung fibroblasts (hLFs) inside the microchannel. The reconfigurable microfluidic device successfully maintained a culture of COS-7 cells in a log phase throughout the entire period of 216 h. Furthermore, we performed a migration assay of cocultured HUVEC and hLF spheroids within one microchannel and observed their migration toward each other.
Figures




References
LinkOut - more resources
Full Text Sources
Other Literature Sources
