Lethal factor antibodies contribute to lethal toxin neutralization in recipients of anthrax vaccine precipitated
- PMID: 28504191
- PMCID: PMC5512426
- DOI: 10.1016/j.vaccine.2017.05.006
Lethal factor antibodies contribute to lethal toxin neutralization in recipients of anthrax vaccine precipitated
Abstract
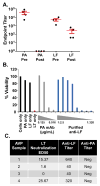
A major difference between two currently licensed anthrax vaccines is presence (United Kingdom Anthrax Vaccine Precipitated, AVP) or absence (United States Anthrax Vaccine Adsorbed, AVA) of quantifiable amounts of the Lethal Toxin (LT) component Lethal Factor (LF). The primary immunogen in both vaccine formulations is Protective Antigen (PA), and LT-neutralizing antibodies directed to PA are an accepted correlate of vaccine efficacy; however, vaccination studies in animal models have demonstrated that LF antibodies can be protective. In this report we compared humoral immune responses in cohorts of AVP (n=39) and AVA recipients (n=78) matched 1:2 for number of vaccinations and time post-vaccination, and evaluated whether the LF response contributes to LT neutralization in human recipients of AVP. PA response rates (≥95%) and PA IgG concentrations were similar in both groups; however, AVP recipients exhibited higher LT neutralization ED50 values (AVP: 1464.0±214.7, AVA: 544.9±83.2, p<0.0001) and had higher rates of LF IgG positivity (95%) compared to matched AVA vaccinees (1%). Multiple regression analysis revealed that LF IgG makes an independent and additive contribution to the LT neutralization response in the AVP group. Affinity purified LF antibodies from two independent AVP recipients neutralized LT and bound to LF Domain 1, confirming contribution of LF antibodies to LT neutralization. This study documents the benefit of including an LF component to PA-based anthrax vaccines.
Keywords: Anthrax; Anthrax vaccine adsorbed; Anthrax vaccine precipitated; Bacillus anthracis; Lethal factor; Lethal toxin; Neutralization; Protective antigen.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures


References
-
- Mock M, Fouet A. Anthrax. Annu Rev Microbiol. 2001;55:647–71. - PubMed
-
- Stanley JL, Smith H. Purification of factor I and recognition of a third factor of the anthrax toxin. J Gen Microbiol. 1961;26:49–63. - PubMed
-
- Bradley KA, Mogridge J, Mourez M, Collier RJ, Young JA. Identification of the cellular receptor for anthrax toxin. Nature. 2001;414:225–9. - PubMed
-
- Klimpel KR, Arora N, Leppla SH. Anthrax toxin lethal factor contains a zinc metalloprotease consensus sequence which is required for lethal toxin activity. Mol Microbiol. 1994;13:1093–100. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

