Low phosphate activates STOP1-ALMT1 to rapidly inhibit root cell elongation
- PMID: 28504266
- PMCID: PMC5440667
- DOI: 10.1038/ncomms15300
Low phosphate activates STOP1-ALMT1 to rapidly inhibit root cell elongation
Abstract
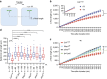
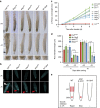
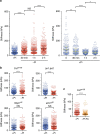
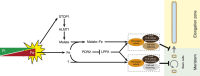
Environmental cues profoundly modulate cell proliferation and cell elongation to inform and direct plant growth and development. External phosphate (Pi) limitation inhibits primary root growth in many plant species. However, the underlying Pi sensory mechanisms are unknown. Here we genetically uncouple two Pi sensing pathways in the root apex of Arabidopsis thaliana. First, the rapid inhibition of cell elongation in the transition zone is controlled by transcription factor STOP1, by its direct target, ALMT1, encoding a malate channel, and by ferroxidase LPR1, which together mediate Fe and peroxidase-dependent cell wall stiffening. Second, during the subsequent slow inhibition of cell proliferation in the apical meristem, which is mediated by LPR1-dependent, but largely STOP1-ALMT1-independent, Fe and callose accumulate in the stem cell niche, leading to meristem reduction. Our work uncovers STOP1 and ALMT1 as a signalling pathway of low Pi availability and exuded malate as an unexpected apoplastic inhibitor of root cell wall expansion.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
-
- Lynch J. P. & Brown K. M. Topsoil foraging—an architectural adaptation of plants to low phosphorus availability. Plant Soil 237, 225–237 (2001).
-
- Lopez-Arredondo D. L., Leyva-Gonzalez M. A., Gonzalez-Morales S. I., Lopez-Bucio J. & Herrera-Estrella L. Phosphate nutrition: improving low-phosphate tolerance in crops. Annu. Rev. Plant Biol. 65, 95–123 (2014). - PubMed
-
- Heidstra R. & Sabatini S. Plant and animal stem cells: similar yet different. Nat. Rev. Mol. Cell. Biol. 15, 301–312 (2014). - PubMed
-
- Sanchez-Calderon L. et al.. Characterization of low phosphorus insensitive mutants reveals a crosstalk between low phosphorus-induced determinate root development and the activation of genes involved in the adaptation of Arabidopsis to phosphorus deficiency. Plant Physiol. 140, 879–889 (2006). - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

