Aerobic capacity mediates susceptibility for the transition from steatosis to steatohepatitis
- PMID: 28504310
- PMCID: PMC5509847
- DOI: 10.1113/JP274281
Aerobic capacity mediates susceptibility for the transition from steatosis to steatohepatitis
Abstract
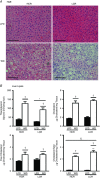
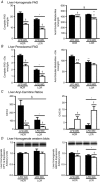
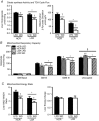
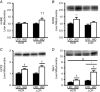
Key points: Low intrinsic aerobic capacity is associated with increased all-cause and liver-related mortality in humans. Low intrinsic aerobic capacity in the low capacity runner (LCR) rat increases susceptibility to acute and chronic high-fat/high-sucrose diet-induced steatosis, without observed increases in liver inflammation. Addition of excess cholesterol to a high-fat/high-sucrose diet produced greater steatosis in LCR and high capacity runner (HCR) rats. However, the LCR rat demonstrated greater susceptibility to increased liver inflammatory and apoptotic markers compared to the HCR rat. The progressive non-alcoholic fatty liver disease observed in the LCR rats following western diet feeding was associated with further declines in liver fatty acid oxidation and mitochondrial respiratory capacity compared to HCR rats.
Abstract: Low aerobic capacity increases risk for non-alcoholic fatty liver disease and liver-related disease mortality, but mechanisms mediating these effects remain unknown. We recently reported that rats bred for low aerobic capacity (low capacity runner; LCR) displayed susceptibility to high fat diet-induced steatosis in association with reduced hepatic mitochondrial fatty acid oxidation (FAO) and respiratory capacity compared to high aerobic capacity (high capacity runner; HCR) rats. Here we tested the impact of aerobic capacity on susceptibility for progressive liver disease following a 16-week 'western diet' (WD) high in fat (45% kcal), cholesterol (1% w/w) and sucrose (15% kcal). Unlike previously with a diet high in fat and sucrose alone, the inclusion of cholesterol in the WD induced hepatomegaly and steatosis in both HCR and LCR rats, while producing greater cholesterol ester accumulation in LCR compared to HCR rats. Importantly, WD-fed low-fitness LCR rats displayed greater inflammatory cell infiltration, serum alanine transaminase, expression of hepatic inflammatory markers (F4/80, MCP-1, TLR4, TLR2 and IL-1β) and effector caspase (caspase 3 and 7) activation compared to HCR rats. Further, LCR rats had greater WD-induced decreases in complete FAO and mitochondrial respiratory capacity. Intrinsic aerobic capacity had no impact on WD-induced hepatic steatosis; however, rats bred for low aerobic capacity developed greater hepatic inflammation, which was associated with reduced hepatic mitochondrial FAO and respiratory capacity and increased accumulation of cholesterol esters. These results confirm epidemiological reports that aerobic capacity impacts progression of liver disease and suggest that these effects are mediated through alterations in hepatic mitochondrial function.
Keywords: aerobic capacity; fatty acid oxidation; inflammation; mitochondria; steatohepatitis.
© 2017 The Authors. The Journal of Physiology © 2017 The Physiological Society.
Figures

Comment in
-
Higher levels of cardiorespiratory fitness keep liver mitochondria happy!J Physiol. 2017 Sep 1;595(17):5719-5720. doi: 10.1113/JP274592. Epub 2017 Aug 2. J Physiol. 2017. PMID: 28730657 Free PMC article. No abstract available.
References
-
- Arguello G, Balboa E, Arrese M & Zanlungo S (2015). Recent insights on the role of cholesterol in non‐alcoholic fatty liver disease. Biochim Biophys Acta 1852, 1765–1778. - PubMed
-
- Bashiri A, Nesan D, Tavallaee G, Sue‐Chue‐Lam I, Chien K, Maguire GF, Naples M, Zhang J, Magomedova L, Adeli K, Cummins CL & Ng DS (2016). Cellular cholesterol accumulation modulates high fat high sucrose (HFHS) diet‐induced ER stress and hepatic inflammasome activation in the development of non‐alcoholic steatohepatitis. Biochim Biophys Acta 1861, 594–605. - PubMed
-
- Begriche K, Massart J, Robin MA, Bonnet F & Fromenty B (2013). Mitochondrial adaptations and dysfunctions in nonalcoholic fatty liver disease. Hepatology 58, 1497–1507. - PubMed
-
- Bernal W, Martin‐Mateos R, Lipcsey M, Tallis C, Woodsford K, McPhail MJ, Willars C, Auzinger G, Sizer E, Heneghan M, Cottam S, Heaton N & Wendon J (2014). Aerobic capacity during cardiopulmonary exercise testing and survival with and without liver transplantation for patients with chronic liver disease. Liver Transpl 20, 54–62. - PubMed
-
- Campbell AM & Chan SH (2007). The voltage dependent anion channel affects mitochondrial cholesterol distribution and function. Arch Biochem Biophys 466, 203–210. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

