Immunotherapy for metastatic renal cell carcinoma
- PMID: 28504837
- PMCID: PMC6484451
- DOI: 10.1002/14651858.CD011673.pub2
Immunotherapy for metastatic renal cell carcinoma
Abstract
Background: Since the mid-2000s, the field of metastatic renal cell carcinoma (mRCC) has experienced a paradigm shift from non-specific therapy with broad-acting cytokines to specific regimens, which directly target the cancer, the tumour microenvironment, or both.Current guidelines recommend targeted therapies with agents such as sunitinib, pazopanib or temsirolimus (for people with poor prognosis) as the standard of care for first-line treatment of people with mRCC and mention non-specific cytokines as an alternative option for selected patients.In November 2015, nivolumab, a checkpoint inhibitor directed against programmed death-1 (PD-1), was approved as the first specific immunotherapeutic agent as second-line therapy in previously treated mRCC patients.
Objectives: To assess the effects of immunotherapies either alone or in combination with standard targeted therapies for the treatment of metastatic renal cell carcinoma and their efficacy to maximize patient benefit.
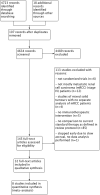
Search methods: We searched the Cochrane Library, MEDLINE (Ovid), Embase (Ovid), ISI Web of Science and registers of ongoing clinical trials in November 2016 without language restrictions. We scanned reference lists and contacted experts in the field to obtain further information.
Selection criteria: We included randomized controlled trials (RCTs) and quasi-RCTs with or without blinding involving people with mRCC.
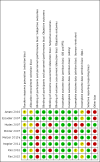
Data collection and analysis: We collected and analyzed studies according to the published protocol. Summary statistics for the primary endpoints were risk ratios (RRs) and mean differences (MD) with their 95% confidence intervals (CIs). We rated the quality of evidence using GRADE methodology and summarized the quality and magnitude of relative and absolute effects for each primary outcome in our 'Summary of findings' tables.
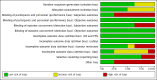
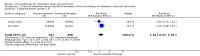
Main results: We identified eight studies with 4732 eligible participants and an additional 13 ongoing studies. We categorized studies into comparisons, all against standard therapy accordingly as first-line (five comparisons) or second-line therapy (one comparison) for mRCC.Interferon (IFN)-α monotherapy probably increases one-year overall mortality compared to standard targeted therapies with temsirolimus or sunitinib (RR 1.30, 95% CI 1.13 to 1.51; 2 studies; 1166 participants; moderate-quality evidence), may lead to similar quality of life (QoL) (e.g. MD -5.58 points, 95% CI -7.25 to -3.91 for Functional Assessment of Cancer - General (FACT-G); 1 study; 730 participants; low-quality evidence) and may slightly increase the incidence of adverse events (AEs) grade 3 or greater (RR 1.17, 95% CI 1.03 to 1.32; 1 study; 408 participants; low-quality evidence).There is probably no difference between IFN-α plus temsirolimus and temsirolimus alone for one-year overall mortality (RR 1.13, 95% CI 0.95 to 1.34; 1 study; 419 participants; moderate-quality evidence), but the incidence of AEs of 3 or greater may be increased (RR 1.30, 95% CI 1.17 to 1.45; 1 study; 416 participants; low-quality evidence). There was no information on QoL.IFN-α alone may slightly increase one-year overall mortality compared to IFN-α plus bevacizumab (RR 1.17, 95% CI 1.00 to 1.36; 2 studies; 1381 participants; low-quality evidence). This effect is probably accompanied by a lower incidence of AEs of grade 3 or greater (RR 0.77, 95% CI 0.71 to 0.84; 2 studies; 1350 participants; moderate-quality evidence). QoL could not be evaluated due to insufficient data.Treatment with IFN-α plus bevacizumab or standard targeted therapy (sunitinib) may lead to similar one-year overall mortality (RR 0.37, 95% CI 0.13 to 1.08; 1 study; 83 participants; low-quality evidence) and AEs of grade 3 or greater (RR 1.18, 95% CI 0.85 to 1.62; 1 study; 82 participants; low-quality evidence). QoL could not be evaluated due to insufficient data.Treatment with vaccines (e.g. MVA-5T4 or IMA901) or standard therapy may lead to similar one-year overall mortality (RR 1.10, 95% CI 0.91 to 1.32; low-quality evidence) and AEs of grade 3 or greater (RR 1.16, 95% CI 0.97 to 1.39; 2 studies; 1065 participants; low-quality evidence). QoL could not be evaluated due to insufficient data.In previously treated patients, targeted immunotherapy (nivolumab) probably reduces one-year overall mortality compared to standard targeted therapy with everolimus (RR 0.70, 95% CI 0.56 to 0.87; 1 study; 821 participants; moderate-quality evidence), probably improves QoL (e.g. RR 1.51, 95% CI 1.28 to 1.78 for clinically relevant improvement of the FACT-Kidney Symptom Index Disease Related Symptoms (FKSI-DRS); 1 study, 704 participants; moderate-quality evidence) and probably reduces the incidence of AEs grade 3 or greater (RR 0.51, 95% CI 0.40 to 0.65; 1 study; 803 participants; moderate-quality evidence).
Authors' conclusions: Evidence of moderate quality demonstrates that IFN-α monotherapy increases mortality compared to standard targeted therapies alone, whereas there is no difference if IFN is combined with standard targeted therapies. Evidence of low quality demonstrates that QoL is worse with IFN alone and that severe AEs are increased with IFN alone or in combination. There is low-quality evidence that IFN-α alone increases mortality but moderate-quality evidence on decreased AEs compared to IFN-α plus bevacizumab. Low-quality evidence shows no difference for IFN-α plus bevacizumab compared to sunitinib with respect to mortality and severe AEs. Low-quality evidence demonstrates no difference of vaccine treatment compared to standard targeted therapies in mortality and AEs, whereas there is moderate-quality evidence that targeted immunotherapies reduce mortality and AEs and improve QoL.
Conflict of interest statement
SU: reported the following financial activities related to the submitted work: institution received support from the Federal Ministry of Education and Research, Germany (grant number: 01KG1024) and from the Wilhelm‐Roux‐Program, Martin Luther University Halle‐Wittenberg, Germany, to write this review.
IM: reported the following financial activities related to the submitted work: institution received support from the Wilhelm‐Roux‐Program, Martin Luther University Halle‐Wittenberg, Germany, for travel to meetings for this review.
MN: none known.
DR: reported the following financial activities related to the submitted work: institution received support from the Wilhelm‐Roux‐Program, Martin Luther University Halle‐Wittenberg, Germany, for travel to meetings for this review.
AVH: none known.
FP: none known.
FG: none known.
BS: none known.
Figures

































Update of
References
References to studies included in this review
-
- Amato RJ, Hawkins RE, Kaufman HL, Thompson JA, Tomczak P, Szczylik C, et al. Vaccination of metastatic renal cancer patients with MVA‐5T4: a randomized, double‐blind, placebo‐controlled phase III study. Clinical Cancer Research 2010;16(22):5539‐47. [DOI: 10.1158/1078-0432.CCR-10-2082] - DOI - PubMed
-
- Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, et al. Temsirolimus, interferon alfa, or both for advanced renal‐cell carcinoma. New England Journal of Medicine2007; Vol. 356, issue 22:2271‐81. - PubMed
-
- Motzer RJ, Hutson TE, Tomczak P, Michaelson D, Bukowski RM, Rixe O, et al. Sunitinib versus interferon alfa in metastatic renal‐cell carcinoma. New England Journal of Medicine 2007;356(2):115‐24. - PubMed
References to studies excluded from this review
-
- Aass N, Mulder PH, Mickisch GH, Mulders P, Oosterom AT, Poppel H, et al. Randomized phase II/III trial of interferon Alfa‐2a with and without 13‐cis‐retinoic acid in patients with progressive metastatic renal cell Carcinoma: the European Organisation for Research and Treatment of Cancer Genito‐Urinary Tract Cancer Group (EORTC 30951). Journal of Clinical Oncology2005; Vol. 23, issue 18:4172‐8. - PubMed
-
- Adler A, Gillon G, Lurie H, Shaham J, Loven D, Shachter Y, et al. Active specific immunotherapy of renal cell carcinoma patients: a prospective randomized study of hormono‐immuno versus hormonotherapy. Preliminary report of immunological and clinical aspects. Journal of Biological Response Modifiers 1987;6(6):610‐24. - PubMed
-
- Amato RJ, Shingler W, Goonewardena M, Belin J, Naylor S, Jac J, et al. Vaccination of renal cell cancer patients with modified vaccinia ankara delivering the tumor antigen 5T4 (TroVax) alone or administered in combination with interferon‐alpha (IFN‐alpha): a phase 2 trial. Journal of Immunotherapy2009; Vol. 32, issue 7:765‐72. - PubMed
-
- Amin A, Dudek AZ, Logan TF, Lance RS, Holzbeierlein JM, Knox JJ, et al. Survival with AGS‐003, an autologous dendritic cell‐based immunotherapy, in combination with sunitinib in unfavourable risk patients with advanced renal cell carcinoma (RCC): phase 2 study results. Journal for Immunotherapy of Cancer 2015;3:14. [DOI: 10.1186/s40425-015-0055-3] - DOI - PMC - PubMed
-
- Atkins MB, Sparano J, Fisher RI, Weiss GR, Margolin KA, Fink KI, et al. Randomized phase II trial of high‐dose interleukin‐2 either alone or in combination with interferon alfa‐2b in advanced renal cell carcinoma. Journal of Clinical Oncology1993; Vol. 11, issue 4:661‐70. - PubMed
References to ongoing studies
-
- AIO‐Studien‐gGmbH. NIVOSWITCH ‐ a randomized phase II study with NIVOlumab or continuation of therapy as an early SWITCH approach in patients with advanced or metastatic renal cell carcinoma (RCC) and disease control after 3 months of treatment with a tyrosine kinase inhibitor, 2016. www.clinicaltrialsregister.eu/ctr‐search/trial/2016‐002170‐13/DE (assessed 5 November 2016).
-
- Figlin RA, Wood CG. ADAPT: an ongoing international phase III randomized trial of autologous dendritic cell immunotherapy (AGS‐003) plus standard treatment in advanced renal cell carcinoma (RCC). Journal of Clinical Oncology 2014;32(Suppl 4):Abstract 449.
-
- Hammers HJ, Plimack E, Sternberg C, McDermott DF, Larkin J, Ravaud A, Rini BI, et al. CheckMate 214: a phase III, randomized, open‐label study of nivolumab combined with ipilimumab versus sunitinib monotherapy in patients with previously untreated metastatic renal cell carcinoma. Journal of Clinical Oncology 2015;33(Suppl):Abstract TPS4578.
-
- NCT0930033. CARMENA: randomized phase III trial evaluating the importance of nephrectomy in patients presenting with metastatic renal cell carcinoma treated with sunitinib, 2009. clinicaltrials.gov/ct2/show/NCT0930033 (assessed 24 March 2016).
-
- NCT01984242. Phase 2 study of atezolizumab (an engineered anti‐PDL1 antibody) as monotherapy or in combination with avastin (bevacizumab) compared to sunitinib in patients with untreated advanced renal cell carcinoma [IMmotion150], 2013. clinicaltrials.gov/ct2/show/NCT01984242 (assessed 24 March 2016).
Additional references
-
- Armstrong AJ, Broderick S, Eisen T, Stadler WM, Jones RJ, Garcia JA, et al. Final clinical results of a randomized phase II international trial of everolimus vs. sunitinib in patients with metastatic non‐clear cell renal cell carcinoma (ASPEN). Journal of Clinical Oncology 2015;15(Suppl 1):Abstract 4507.
-
- Athar U, Gentile TC. Treatment options for metastatic renal cell carcinoma: a review. Canadian Journal of Urology 2008;15(2):3954‐66. - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

