Real-time crystallographic studies of the adenine riboswitch using an X-ray free-electron laser
- PMID: 28504865
- PMCID: PMC6309305
- DOI: 10.1111/febs.14110
Real-time crystallographic studies of the adenine riboswitch using an X-ray free-electron laser
Abstract
Structures of the four reaction states of the adenine riboswitch aptamer domain, including a transient intermediate state were solved by serial femtosecond crystallography. The structures not only demonstrate the use of X-ray free-electron lasers for RNA crystallography but have also proven that transient states can be determined in real time by mix-and-inject crystallography. These results illustrate the structural basis for the ligand-induced conformational changes associated with the molecular 'switch'.
Keywords: RNA; X-ray free-electron laser; adenine riboswitch; diffusive mixing; lattice conversion; phase transition; reaction intermediate; serial femtosecond crystallography; time-resolved crystallography.
Published 2017. This article is a U.S. Government work and is in the public domain in the USA.
Figures
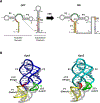

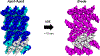
Similar articles
-
Structures of riboswitch RNA reaction states by mix-and-inject XFEL serial crystallography.Nature. 2017 Jan 12;541(7636):242-246. doi: 10.1038/nature20599. Epub 2016 Nov 14. Nature. 2017. PMID: 27841871 Free PMC article.
-
Mix-and-Inject Serial Femtosecond Crystallography to Capture RNA Riboswitch Intermediates.Methods Mol Biol. 2023;2568:243-249. doi: 10.1007/978-1-0716-2687-0_16. Methods Mol Biol. 2023. PMID: 36227573
-
Conformational flexibility of adenine riboswitch aptamer in apo and bound states using NMR and an X-ray free electron laser.J Biomol NMR. 2019 Sep;73(8-9):509-518. doi: 10.1007/s10858-019-00278-w. Epub 2019 Oct 12. J Biomol NMR. 2019. PMID: 31606878 Free PMC article.
-
[The adenine riboswitch: a new gene regulation mechanism].Med Sci (Paris). 2006 Dec;22(12):1053-9. doi: 10.1051/medsci/200622121053. Med Sci (Paris). 2006. PMID: 17156726 Review. French.
-
[What Kind of Measurements Can Be Made with an X-ray Free Electron Laser at SACLA?].Yakugaku Zasshi. 2022;142(5):479-485. doi: 10.1248/yakushi.21-00203-1. Yakugaku Zasshi. 2022. PMID: 35491153 Review. Japanese.
Cited by
-
Dynamic Structural Biology Experiments at XFEL or Synchrotron Sources.Methods Mol Biol. 2021;2305:203-228. doi: 10.1007/978-1-0716-1406-8_11. Methods Mol Biol. 2021. PMID: 33950392 Review.
-
Tying the knot in the tetrahydrofolate (THF) riboswitch: A molecular basis for gene regulation.J Struct Biol. 2021 Mar;213(1):107703. doi: 10.1016/j.jsb.2021.107703. Epub 2021 Feb 9. J Struct Biol. 2021. PMID: 33571639 Free PMC article.
-
Co-crystal structure of the iMango-III fluorescent RNA aptamer using an X-ray free-electron laser.Acta Crystallogr F Struct Biol Commun. 2019 Aug 1;75(Pt 8):547-551. doi: 10.1107/S2053230X19010136. Epub 2019 Aug 2. Acta Crystallogr F Struct Biol Commun. 2019. PMID: 31397326 Free PMC article.
-
Scaffold-enabled high-resolution cryo-EM structure determination of RNA.bioRxiv [Preprint]. 2024 Jun 10:2024.06.10.598011. doi: 10.1101/2024.06.10.598011. bioRxiv. 2024. Update in: Nat Commun. 2025 Jan 21;16(1):880. doi: 10.1038/s41467-024-55699-5. PMID: 38915706 Free PMC article. Updated. Preprint.
-
Scaffold-enabled high-resolution cryo-EM structure determination of RNA.Nat Commun. 2025 Jan 21;16(1):880. doi: 10.1038/s41467-024-55699-5. Nat Commun. 2025. PMID: 39837824 Free PMC article.
References
-
- Nahvi A, Sudarsan N, Ebert MS, Zou X, Brown KL & Breaker RR (2002) Genetic control by a metabolite binding mRNA, Chem Biol. 9, 1043. - PubMed
-
- Winkler WC & Breaker RR (2003) Genetic control by metabolite-binding riboswitches, Chembiochem. 4, 1024–32. - PubMed
-
- Stagno JR, Liu Y, Bhandari YR, Conrad CE, Panja S, Swain M, Fan L, Nelson G, Li C, Wendel DR, White TA, Coe JD, Wiedorn MO, Knoska J, Oberthuer D, Tuckey RA, Yu P, Dyba M, Tarasov SG, Weierstall U, Grant TD, Schwieters CD, Zhang J, Ferre-D’Amare AR, Fromme P, Draper DE, Liang M, Hunter MS, Boutet S, Tan K, Zuo X, Ji X, Barty A, Zatsepin NA, Chapman HN, Spence JC, Woodson SA & Wang YX (2016) Structures of riboswitch RNA reaction states by mix-and-inject XFEL serial crystallography, Nature. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

