Structure-Activity Relationships of Bioengineered Heparin/Heparan Sulfates Produced in Different Bioreactors
- PMID: 28505124
- PMCID: PMC6154572
- DOI: 10.3390/molecules22050806
Structure-Activity Relationships of Bioengineered Heparin/Heparan Sulfates Produced in Different Bioreactors
Abstract
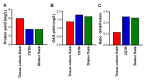
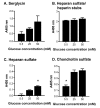
Heparin and heparan sulfate are structurally-related carbohydrates with therapeutic applications in anticoagulation, drug delivery, and regenerative medicine. This study explored the effect of different bioreactor conditions on the production of heparin/heparan sulfate chains via the recombinant expression of serglycin in mammalian cells. Tissue culture flasks and continuously-stirred tank reactors promoted the production of serglycin decorated with heparin/heparan sulfate, as well as chondroitin sulfate, while the serglycin secreted by cells in the tissue culture flasks produced more highly-sulfated heparin/heparan sulfate chains. The serglycin produced in tissue culture flasks was effective in binding and signaling fibroblast growth factor 2, indicating the utility of this molecule in drug delivery and regenerative medicine applications in addition to its well-known anticoagulant activity.
Keywords: bioreactor; heparan sulfate; heparin; proteoglycan; recombinant expression; serglycin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Optimization of bioengineered heparin/heparan sulfate production for therapeutic applications.Bioengineered. 2017 Sep 3;8(5):661-664. doi: 10.1080/21655979.2017.1301328. Epub 2017 Apr 10. Bioengineered. 2017. PMID: 28394734 Free PMC article.
-
Platelet Factor 4 Binds to Vascular Proteoglycans and Controls Both Growth Factor Activities and Platelet Activation.J Biol Chem. 2017 Mar 10;292(10):4054-4063. doi: 10.1074/jbc.M116.760660. Epub 2017 Jan 23. J Biol Chem. 2017. PMID: 28115521 Free PMC article.
-
Bioengineered human heparin with anticoagulant activity.Metab Eng. 2016 Nov;38:105-114. doi: 10.1016/j.ymben.2016.07.006. Epub 2016 Jul 18. Metab Eng. 2016. PMID: 27445159
-
Mast cell glycosaminoglycans.Glycoconj J. 2017 Jun;34(3):351-361. doi: 10.1007/s10719-016-9749-0. Epub 2016 Nov 30. Glycoconj J. 2017. PMID: 27900574 Free PMC article. Review.
-
Use of biosynthetic enzymes in heparin and heparan sulfate synthesis.Bioorg Med Chem. 2013 Aug 15;21(16):4786-92. doi: 10.1016/j.bmc.2012.11.053. Epub 2012 Dec 12. Bioorg Med Chem. 2013. PMID: 23313092 Review.
Cited by
-
Molecular forms of neurogranin in cerebrospinal fluid.J Neurochem. 2021 May;157(3):816-833. doi: 10.1111/jnc.15252. Epub 2020 Dec 17. J Neurochem. 2021. PMID: 33249594 Free PMC article.
-
Multifaceted Heparin: Diverse Applications beyond Anticoagulant Therapy.Pharmaceuticals (Basel). 2024 Oct 12;17(10):1362. doi: 10.3390/ph17101362. Pharmaceuticals (Basel). 2024. PMID: 39459002 Free PMC article. Review.
-
Looking Forward to the Future of Heparin: New Sources, Developments and Applications.Molecules. 2018 Jan 31;23(2):293. doi: 10.3390/molecules23020293. Molecules. 2018. PMID: 29385025 Free PMC article.
-
Non-Anticoagulant Heparan Sulfate from the Ascidian Phallusia nigra Prevents Colon Carcinoma Metastasis in Mice by Disrupting Platelet-Tumor Cell Interaction.Cancers (Basel). 2020 May 26;12(6):1353. doi: 10.3390/cancers12061353. Cancers (Basel). 2020. PMID: 32466418 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

