Upregulated Autophagy in Sertoli Cells of Ethanol-Treated Rats Is Associated with Induction of Inducible Nitric Oxide Synthase (iNOS), Androgen Receptor Suppression and Germ Cell Apoptosis
- PMID: 28505146
- PMCID: PMC5454973
- DOI: 10.3390/ijms18051061
Upregulated Autophagy in Sertoli Cells of Ethanol-Treated Rats Is Associated with Induction of Inducible Nitric Oxide Synthase (iNOS), Androgen Receptor Suppression and Germ Cell Apoptosis
Abstract
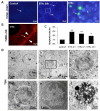
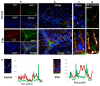
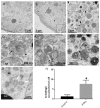
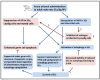
This study was conducted to investigate the autophagic response of Sertoli cells (SCs) to acute ethanol toxicity using in vivo and in vitro models. Adult Wistar rats were intraperitoneally injected with either 5 g/kg ethanol or phosphate-buffered saline (for the control group) and sacrificed 0, 3, 6 and 24 h after injection. Compared to the control group, enhanced germ cell apoptosis was observed in the ethanol-treated rats (ETRs) in association with upregulation of iNOS and reduced expression of androgen receptor protein levels in SCs, which were resistant to apoptosis. Meanwhile, autophagy was upregulated in ETR SCs (peaking at 24 h) compared to the control group, as evidenced by transcription factor EB (TFEB) nuclear translocation, enhanced expression of microtubule-associated protein 1 light chain3-II (LC3-II), lysosome-associated membrane protein-2 (LAMP-2), pan cathepsin protein levels and reduced expression of p62. This upregulation of SC autophagy was confirmed ultrastructurally by enhanced formation of autophagic vacuoles and by immunofluorescent double labelling of autophagosomal and lysosomal markers. Study of cultured SCs confirmed enhanced autophagic response to ethanol toxicity, which was cytoprotective based on decreased viability of SCs upon blocking autophagy with 3-methyladenine (3-MA). The results highlighted the molecular mechanisms of prosurvival autophagy in ETR SCs for the first time, and may have significant implications for male fertility.
Keywords: Sertoli; androgen receptor (AR); apoptosis; autophagy; ethanol; inducible nitric oxide synthase (iNOS); transcription factor EB (TFEB).
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Rato L., Meneses M.J, Silva B.M., Sousa M., Alves M.G., Oliveira P.F. New insights on hormones and factors that modulate Sertoli cell metabolism. Histol. Histopathol. 2016;31:499–513. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

