Rapid deployment of a mobile biosafety level-3 laboratory in Sierra Leone during the 2014 Ebola virus epidemic
- PMID: 28505171
- PMCID: PMC5444861
- DOI: 10.1371/journal.pntd.0005622
Rapid deployment of a mobile biosafety level-3 laboratory in Sierra Leone during the 2014 Ebola virus epidemic
Abstract
Background: Ebola virus emerged in West Africa in December 2013. The high population mobility and poor public health infrastructure in this region led to the development of the largest Ebola virus disease (EVD) outbreak to date.
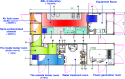
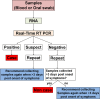
Methodology/principal findings: On September 26, 2014, China dispatched a Mobile Biosafety Level-3 Laboratory (MBSL-3 Lab) and a well-trained diagnostic team to Sierra Leone to assist in EVD diagnosis using quantitative real-time PCR, which allowed the diagnosis of suspected EVD cases in less than 4 hours from the time of sample receiving. This laboratory was composed of three container vehicles equipped with advanced ventilation system, communication system, electricity and gas supply system. We strictly applied multiple safety precautions to reduce exposure risks. Personnel, materials, water and air flow management were the key elements of the biosafety measures in the MBSL-3 Lab. Air samples were regularly collected from the MBSL-3 Lab, but no evidence of Ebola virus infectious aerosols was detected. Potentially contaminated objects were also tested by collecting swabs. On one occasion, a pipette tested positive for EVD. A total of 1,635 suspected EVD cases (824 positive [50.4%]) were tested from September 28 to November 11, 2014, and no member of the diagnostic team was infected with Ebola virus or other pathogens, including Lassa fever. The specimens tested included blood (69.2%) and oral swabs (30.8%) with positivity rates of 54.2% and 41.9%, respectively. The China mobile laboratory was thus instrumental in the EVD outbreak response by providing timely and reliable diagnostics.
Conclusions/significance: The MBSL-3 Lab significantly contributed to establishing a suitable laboratory response capacity during the emergence of EVD in Sierra Leone.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Kuhn JH, Becker S, Ebihara H, Geisbert TW, Johnson KM, Kawaoka Y,et al. Proposal for a revised taxonomy of the family Filoviridae: classification, names of taxa and viruses, and virus abbreviations. Arch Virol. 2010. December;155(12):2083–103. doi: 10.1007/s00705-010-0814-x - DOI - PMC - PubMed
-
- Sanchez AG, Thomas W, Feldmann H. Filoviridae: Marburg and Ebola viruses In: Knipe DM, Fields BN, Howley PM, editors. Fields Virology. 5th ed. Philadelphia: Lippincott Williams and Wilkins; 2007. p.1409–48
-
- Sullivan N, Yang ZY, Nabel GJ. Ebola virus pathogenesis: Implications for vaccines and therapies. J Virol. 2003. September;77(18):9733–7. doi: 10.1128/JVI.77.18.9733-9737.2003 - DOI - PMC - PubMed
-
- Bray M, Geisbert TW. Ebola virus: the role of macrophages and dendritic cells in the pathogenesis of Ebola hemorrhagic fever. Int J Biochem Cell Biol. 2005. August;37(8):1560–6. doi: 10.1016/j.biocel.2005.02.018 - DOI - PubMed
-
- Centers for Disease Control and Prevention. Ebola Hemorrhagic Fever Signs and Symptoms [Internet]. 2014. Available from: http://www.cdc.gov/vhf/ebola/symptoms/.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

