Extracellular matrix nitration alters growth factor release and activates bioactive complement in human retinal pigment epithelial cells
- PMID: 28505174
- PMCID: PMC5432172
- DOI: 10.1371/journal.pone.0177763
Extracellular matrix nitration alters growth factor release and activates bioactive complement in human retinal pigment epithelial cells
Abstract
Purpose: We have shown previously that non-enzymatic nitration (NEN) of the extracellular matrix (ECM), which serves as a model of Bruch's membrane (BM) aging, has a profound effect on the behavior of the overlying retinal pigment epithelial (RPE) cells, including altered phagocytic ability, reduced cell adhesion, and inhibition of proliferation. We know that transplanted RPE monolayers will encounter a hostile sub-RPE environment, including age-related alterations in BM that may compromise cell function and survival. Here we use our previous NEN model of BM aging to determine the effects of NEN of the ECM on growth factor release and complement activation in RPE cells.
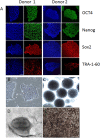
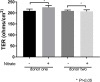
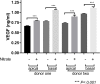
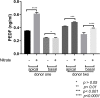
Methods: Human induced-pluripotent stem cells (iPSCs) were differentiated into RPE cells, and confirmed by immunohistochemistry, confocal microscopy, and polymerase chain reaction. IPSC-derived RPE cells were plated onto RPE-derived ECM under untreated or nitrite-modified conditions. Cells were cultured for 7 days and barrier function measured by transepithelial resistance (TER). Vascular endothelial growth factor (VEGF), pigment epithelium-derived factor (PEDF), and complement component C3a were measured using enzyme-linked immunosorbent assay (ELISA).
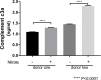
Results: On average nitrite-modified ECM increased VEGF release both apically and basally by 0.15 ± 0.014 ng/mL (p <0.0001) and 0.21 ± 0.022 ng/mL (p <0.0001), respectively, in iPSC-derived RPE cells. Nitrite-modified ECM increased PEDF release in iPSC-derived RPE cells apically by 0.16 ± 0.031 ng/mL (p <0.0001), but not basally (0.27 ± 0.015 vs. 0.32 ± 0.029 ng/mL, (p >0.05)). Nitrite-modified ECM increased production of C3a in iPSC-derived RPE cells by 0.52 ± 0.123 ng/mL (p <0.05).
Conclusion: Nitrite-modified ECM increased VEGF, PEDF release, and C3a production in human iPSC-derived RPE cells. This model demonstrates changes seen in the basement membrane can lead to alterations in the cell biology of the RPE cells that may be related to the development of age-related macular degeneration.
Conflict of interest statement
Figures

Similar articles
-
Stem cell-derived retinal pigment epithelium from patients with age-related macular degeneration exhibit reduced metabolism and matrix interactions.Stem Cells Transl Med. 2020 Mar;9(3):364-376. doi: 10.1002/sctm.19-0321. Epub 2019 Dec 16. Stem Cells Transl Med. 2020. PMID: 31840941 Free PMC article.
-
Attainment of polarity promotes growth factor secretion by retinal pigment epithelial cells: relevance to age-related macular degeneration.Aging (Albany NY). 2009 Dec 27;2(1):28-42. doi: 10.18632/aging.100111. Aging (Albany NY). 2009. PMID: 20228934 Free PMC article.
-
Nitrite Modification of Extracellular Matrix Alters CD46 Expression and VEGF Release in Human Retinal Pigment Epithelium.Invest Ophthalmol Vis Sci. 2015 Jul;56(8):4231-8. doi: 10.1167/iovs.15-16438. Invest Ophthalmol Vis Sci. 2015. PMID: 26161984 Free PMC article.
-
Retinoid Processing in Induced Pluripotent Stem Cell-Derived Retinal Pigment Epithelium Cultures.Prog Mol Biol Transl Sci. 2015;134:477-90. doi: 10.1016/bs.pmbts.2015.06.004. Epub 2015 Jul 9. Prog Mol Biol Transl Sci. 2015. PMID: 26310172 Free PMC article. Review.
-
Immunological aspects of RPE cell transplantation.Prog Retin Eye Res. 2021 Sep;84:100950. doi: 10.1016/j.preteyeres.2021.100950. Epub 2021 Jan 19. Prog Retin Eye Res. 2021. PMID: 33482342 Review.
Cited by
-
Impaired Mitochondrial Function in iPSC-Retinal Pigment Epithelium with the Complement Factor H Polymorphism for Age-Related Macular Degeneration.Cells. 2021 Apr 2;10(4):789. doi: 10.3390/cells10040789. Cells. 2021. PMID: 33918210 Free PMC article.
-
The extracellular microenvironment in immune dysregulation and inflammation in retinal disorders.Front Immunol. 2023 Mar 1;14:1147037. doi: 10.3389/fimmu.2023.1147037. eCollection 2023. Front Immunol. 2023. PMID: 36936905 Free PMC article. Review.
-
The Long Pentraxin PTX3 as a New Biomarker and Pharmacological Target in Age-Related Macular Degeneration and Diabetic Retinopathy.Front Pharmacol. 2022 Jan 7;12:811344. doi: 10.3389/fphar.2021.811344. eCollection 2021. Front Pharmacol. 2022. PMID: 35069222 Free PMC article. Review.
-
Stem cell-derived retinal pigment epithelium from patients with age-related macular degeneration exhibit reduced metabolism and matrix interactions.Stem Cells Transl Med. 2020 Mar;9(3):364-376. doi: 10.1002/sctm.19-0321. Epub 2019 Dec 16. Stem Cells Transl Med. 2020. PMID: 31840941 Free PMC article.
-
Neovascular Macular Degeneration: A Review of Etiology, Risk Factors, and Recent Advances in Research and Therapy.Int J Mol Sci. 2021 Jan 25;22(3):1170. doi: 10.3390/ijms22031170. Int J Mol Sci. 2021. PMID: 33504013 Free PMC article. Review.
References
-
- Wiradjaja F, DiTommaso T, Smyth I. Basement membranes in development and disease. Birth Defects Res C Embryo Today. 2010;90(1):8–31. doi: 10.1002/bdrc.20172 - DOI - PubMed
-
- Nystrom A, Bornert O, Kuhl T. Cell therapy for basement membrane-linked diseases. Matrix Biol. 2016. - PubMed
-
- Pauleikhoff D, Harper CA, Marshall J, Bird AC. Aging changes in Bruch's membrane. A histochemical and morphologic study. Ophthalmology. 1990;97(2):171–8. Epub 1990/02/01. - PubMed
-
- Pauleikhoff D, Harper CA, Marshall J, Bird AC. Aging changes in Bruch's membrane. A histochemical and morphologic study. Ophthalmology. 1990;97(2):171–8. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

