Effect of Combined Patient Decision Aid and Patient Navigation vs Usual Care for Colorectal Cancer Screening in a Vulnerable Patient Population: A Randomized Clinical Trial
- PMID: 28505217
- PMCID: PMC5710456
- DOI: 10.1001/jamainternmed.2017.1294
Effect of Combined Patient Decision Aid and Patient Navigation vs Usual Care for Colorectal Cancer Screening in a Vulnerable Patient Population: A Randomized Clinical Trial
Erratum in
-
Errors in Figure and Funding/Support Paragraph.JAMA Intern Med. 2017 Jul 1;177(7):1062. doi: 10.1001/jamainternmed.2017.3162. JAMA Intern Med. 2017. PMID: 28672349 Free PMC article. No abstract available.
Abstract
Importance: Colorectal cancer (CRC) screening is underused, especially among vulnerable populations. Decision aids and patient navigation are potentially complementary interventions for improving CRC screening rates, but their combined effect on screening completion is unknown.
Objective: To determine the combined effect of a CRC screening decision aid and patient navigation compared with usual care on CRC screening completion.
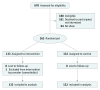
Design, setting, and participants: In this randomized clinical trial, data were collected from January 2014 to March 2016 at 2 community health center practices, 1 in North Carolina and 1 in New Mexico, serving vulnerable populations. Patients ages 50 to 75 years who had average CRC risk, spoke English or Spanish, were not current with recommended CRC screening, and were attending primary care visits were recruited and randomized 1:1 to intervention or control arms.
Interventions: Intervention participants viewed a CRC screening decision aid in English or Spanish immediately before their clinician encounter. The decision aid promoted screening and presented colonoscopy and fecal occult blood testing as screening options. After the clinician encounter, intervention patients received support for screening completion from a bilingual patient navigator. Control participants viewed a food safety video before the encounter and otherwise received usual care.
Main outcomes and measures: The primary outcome was CRC screening completion within 6 months of the index study visit assessed by blinded medical record review.
Results: Characteristics of the 265 participants were as follows: their mean age was 58 years; 173 (65%) were female, 164 (62%) were Latino; 40 (15%) were white non-Latino; 61 (23%) were black or of mixed race; 191 (78%) had a household income of less than $20 000; 101 (38%) had low literacy; 75 (28%) were on Medicaid; and 91 (34%) were uninsured. Intervention participants were more likely to complete CRC screening within 6 months (68% vs 27%); adjusted-difference, 40 percentage points (95% CI, 29-51 percentage points). The intervention was more effective in women than in men (50 vs 21 percentage point increase, interaction P = .02). No effect modification was observed across other subgroups.
Conclusions and relevance: A patient decision aid plus patient navigation increased the rate of CRC screening completion in compared with usual care invulnerable primary care patients.
Trial registration: clinicaltrials.gov Identifier: NCT02054598.
Conflict of interest statement
Figures
References
-
- Siegel RL, Miller KD, Jamal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5-29. - PubMed
-
- American Cancer Society Cancer Facts & Figures 2016. Atlanta, GA: American Cancer Society; 2016.
-
- U.S. Preventive Services Task Force Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149(9):627-637. - PubMed
-
- Bibbins-Domingo K, Grossman DC, Curry SJ, et al. ; US Preventive Services Task Force . Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2016;315(23):2564-2575. - PubMed
-
- Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM; American College of Gastroenterology . American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected]. Am J Gastroenterol. 2009;104(3):739-750. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical