Sialic acid deficiency is associated with oxidative stress leading to muscle atrophy and weakness in GNE myopathy
- PMID: 28505249
- PMCID: PMC6075185
- DOI: 10.1093/hmg/ddx192
Sialic acid deficiency is associated with oxidative stress leading to muscle atrophy and weakness in GNE myopathy
Abstract
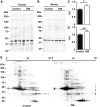
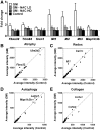
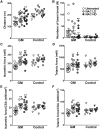
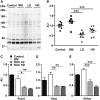
Sialic acids are monosaccharides found in terminal sugar chains of cell surfaces and proteins; they have various biological functions and have been implicated in health and disease. Genetic defects of the GNE gene which encodes a critical bifunctional enzyme for sialic acid biosynthesis, lead to GNE myopathy, a disease manifesting with progressive muscle atrophy and weakness. The likely mechanism of disease is a lack of sialic acids. There remains, however, an unexplained link between hyposialylation and the muscle atrophy and weakness. In this study, we found that muscle proteins were highly modified by S-nitrosylation, and that oxidative stress-responsive genes were significantly upregulated, in hyposialylated muscles from human GNE myopathy patients and model mice. In both in vitro and in vivo models, the production of reactive oxygen species (ROS) was elevated with cellular hyposialylation, and increasing overall sialylation by extrinsic sialic acid intake reduced ROS and protein S-nitrosylation. More importantly, the antioxidant, oral N-acetylcysteine led to amelioration of the muscle atrophy and weakness in Gne mutant mice. Our data provide evidence of additional important function of sialic acids as a ROS scavenger in skeletal muscles, expanding our understanding on how sialic acid deficiency contributes to disease pathology, and identify oxidative stress as a therapeutic target in GNE myopathy.
© The Author 2017. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

