Potential reward reduces the adverse impact of negative distractor stimuli
- PMID: 28505380
- PMCID: PMC5629819
- DOI: 10.1093/scan/nsx067
Potential reward reduces the adverse impact of negative distractor stimuli
Abstract
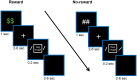
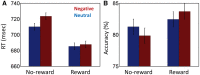
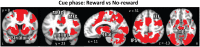
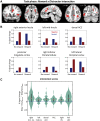
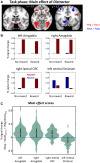
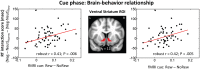
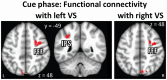
Knowledge about interactions between reward and negative processing is rudimentary. Here, we employed functional MRI to probe how potential reward signaled by advance cues alters aversive distractor processing during perception. Behaviorally, the influence of aversive stimuli on task performance was reduced during the reward compared to no-reward condition. In the brain, at the task phase, paralleling the observed behavioral pattern, we observed significant interactions in the anterior insula and dorsal anterior cingulate cortex, such that responses during the negative (vs neutral) condition were reduced during the reward compared to no-reward condition. Notably, negative distractor processing in the amygdala appeared to be independent of the reward manipulation. During the initial cue phase, we observed increased reward-related responses in the ventral striatum/accumbens, which were correlated with behavioral interference scores at the subsequent task phase, revealing that participants with increased reward-related responses exhibited a greater behavioral benefit of reward in reducing the adverse effect of negative images. Furthermore, during processing of reward (vs no-reward) cues, the ventral striatum exhibited stronger functional connectivity with fronto-parietal regions important for attentional control. Together, our findings contribute to the understanding of how potential reward influences attentional control and reduces negative distractor processing in the human brain.
Keywords: amygdala; emotion; perception; reward; ventral striatum.
© The Author (2017). Published by Oxford University Press. For Permissions, please email: journals.permissions@oup.com.
Figures
References
-
- Boehler C.N., Hopf J.M., Stoppel C.M., Krebs R.M. (2012). Motivating inhibition: reward prospect speeds up response cancellation. Cognition, 125, 498–503. - PubMed
-
- Botvinick M., Braver T. (2015). Motivation and cognitive control: from behavior to neural mechanism. Psychology, 66, 83. - PubMed
-
- Braem S., Verguts T., Roggeman C., Notebaert W. (2012). Reward modulates adaptations to conflict. Cognition, 125, 324–32. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

