Dihydroartemisinin inhibits TCTP-dependent metastasis in gallbladder cancer
- PMID: 28506239
- PMCID: PMC5433060
- DOI: 10.1186/s13046-017-0531-3
Dihydroartemisinin inhibits TCTP-dependent metastasis in gallbladder cancer
Erratum in
-
Correction to: Dihydroartemisinin inhibits TCTP-dependent metastasis in gallbladder cancer.J Exp Clin Cancer Res. 2019 Sep 3;38(1):385. doi: 10.1186/s13046-019-1378-6. J Exp Clin Cancer Res. 2019. PMID: 31481102 Free PMC article.
-
Correction to: Dihydroartemisinin inhibits TCTPdependent metastasis in gallbladder cancer.J Exp Clin Cancer Res. 2022 Apr 4;41(1):124. doi: 10.1186/s13046-022-02325-1. J Exp Clin Cancer Res. 2022. PMID: 35369879 Free PMC article. No abstract available.
Abstract
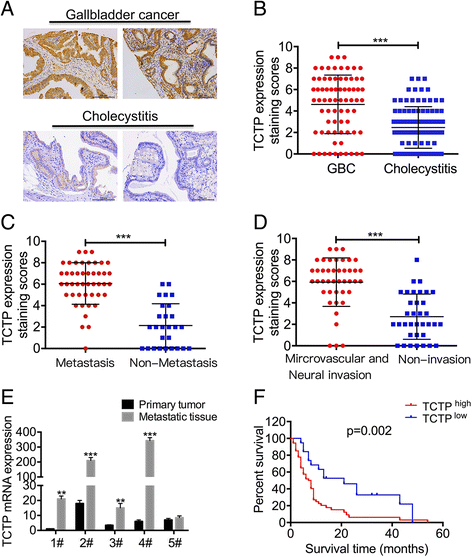
Background: Patients with metastatic or relapsed gallbladder cancer generally have a poor prognosis. Therefore, targeting metastasis is one arm of therapeutic strategies to treat gallbladder cancer.
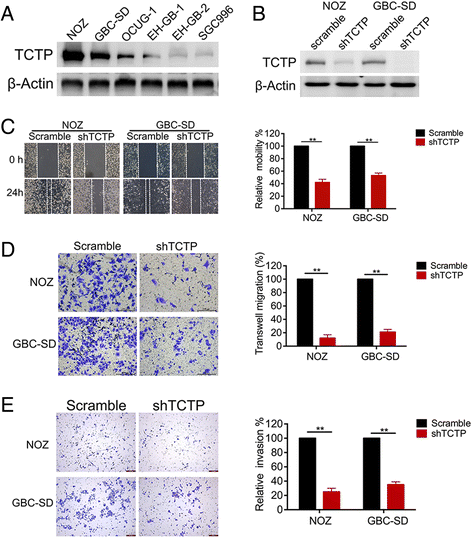
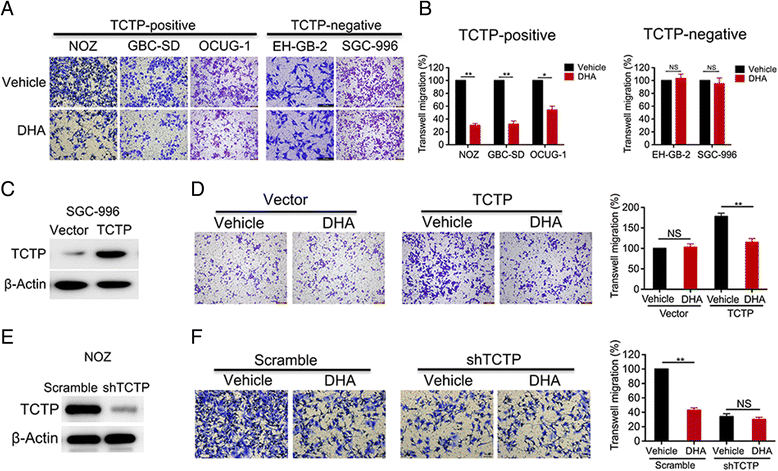
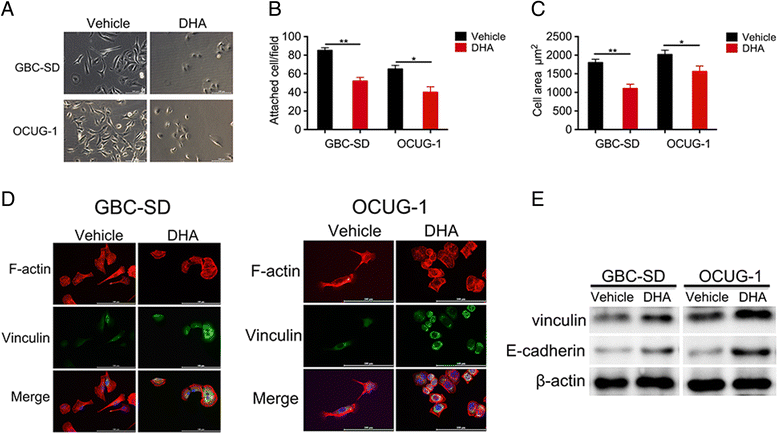
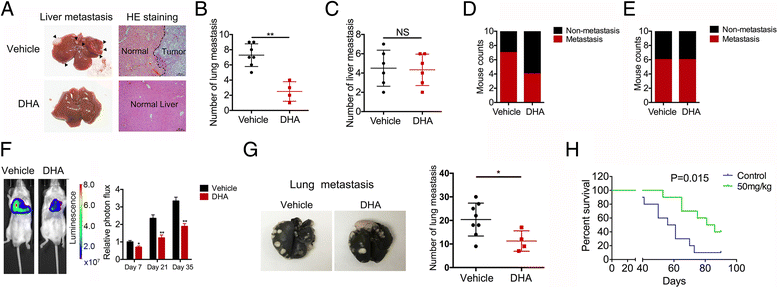
Methods: Levels of translationally controlled tumor protein (TCTP) were measured in samples of gallbladder cancer by immunohistochemical staining. Wound healing, migration and invasion assays were used to investigate the motility of cells. Western blot assay was used to investigate the levels of TCTP and other proteins. Liver metastasis models and lung metastasis models were established to investigate the inhibitory effect of Dihydroartemisinin on gallbladder cancer metastasis.
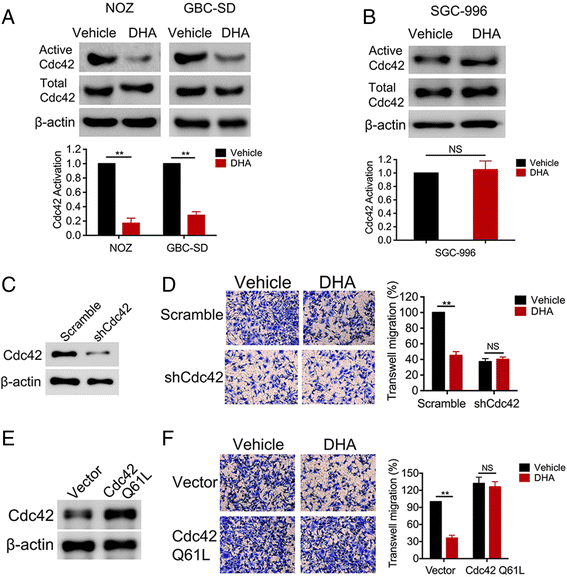
Results: TCTP is aberrantly expressed in gallbladder cancer patients and associated with metastasis and a poor prognosis. Depleting TCTP significantly inhibited gallbladder cancer cell migration and invasion. We found that Dihydroartemisinin as a potent inhibitor of TCTP inhibited TCTP-dependent cell migration and invasion by reducing cell division control protein 42 homolog (Cdc42) activation. In addition, in mice with xenografted tumors, treatment with Dihydroartemisinin decreased gallbladder cancer cell metastases and improved survival.
Conclusions: These findings provide new insights into the therapeutic activity of Dihydroartemisinin as a treatment for gallbladder cancer metastasis.
Keywords: Dihydroartemisinin; Gallbladder cancer; Invasion; Metastasis; TCTP.
Figures
Similar articles
-
Combination of dihydroartemisinin and resveratrol effectively inhibits cancer cell migration via regulation of the DLC1/TCTP/Cdc42 pathway.Food Funct. 2020 Nov 18;11(11):9573-9584. doi: 10.1039/d0fo00996b. Food Funct. 2020. PMID: 33150340
-
Extracellular translationally controlled tumor protein promotes colorectal cancer invasion and metastasis through Cdc42/JNK/ MMP9 signaling.Oncotarget. 2016 Aug 2;7(31):50057-50073. doi: 10.18632/oncotarget.10315. Oncotarget. 2016. PMID: 27367023 Free PMC article.
-
TROP2 promotes proliferation, migration and metastasis of gallbladder cancer cells by regulating PI3K/AKT pathway and inducing EMT.Oncotarget. 2017 Jul 18;8(29):47052-47063. doi: 10.18632/oncotarget.16789. Oncotarget. 2017. PMID: 28423362 Free PMC article.
-
Translationally controlled tumor protein: the mediator promoting cancer invasion and migration and its potential clinical prospects.J Zhejiang Univ Sci B. 2022 Aug 15;23(8):642-654. doi: 10.1631/jzus.B2100910. J Zhejiang Univ Sci B. 2022. PMID: 35953758 Free PMC article. Review.
-
Protein transduction domain of translationally controlled tumor protein: characterization and application in drug delivery.Drug Deliv. 2022 Dec;29(1):3009-3021. doi: 10.1080/10717544.2022.2122636. Drug Deliv. 2022. PMID: 36104954 Free PMC article. Review.
Cited by
-
SLC25A22 Promotes Proliferation and Metastasis of Osteosarcoma Cells via the PTEN Signaling Pathway.Technol Cancer Res Treat. 2018 Jan 1;17:1533033818811143. doi: 10.1177/1533033818811143. Technol Cancer Res Treat. 2018. PMID: 30482097 Free PMC article.
-
Ferroptosis: The Silver Lining of Cancer Therapy.Front Cell Dev Biol. 2021 Nov 29;9:765859. doi: 10.3389/fcell.2021.765859. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34912804 Free PMC article. Review.
-
DHA Affects Microtubule Dynamics Through Reduction of Phospho-TCTP Levels and Enhances the Antiproliferative Effect of T-DM1 in Trastuzumab-Resistant HER2-Positive Breast Cancer Cell Lines.Cells. 2020 May 19;9(5):1260. doi: 10.3390/cells9051260. Cells. 2020. PMID: 32438775 Free PMC article.
-
Rapamycin promotes the anticancer action of dihydroartemisinin in breast cancer MDA-MB-231 cells by regulating expression of Atg7 and DAPK.Oncol Lett. 2018 Apr;15(4):5781-5786. doi: 10.3892/ol.2018.8013. Epub 2018 Feb 9. Oncol Lett. 2018. PMID: 29545903 Free PMC article.
-
Preoperative serum fibrinogen as a valuable predictor in the nomogram predicting overall survival of postoperative patients with gallbladder cancer.J Gastrointest Oncol. 2021 Aug;12(4):1661-1672. doi: 10.21037/jgo-21-357. J Gastrointest Oncol. 2021. PMID: 34532118 Free PMC article.
References
-
- Shu YJ, Weng H, Ye YY, Hu YP, Bao RF, Cao Y, Wang XA, Zhang F, Xiang SS, Li HF, et al. SPOCK1 as a potential cancer prognostic marker promotes the proliferation and metastasis of gallbladder cancer cells by activating the PI3K/AKT pathway. Mol Cancer. 2015;14:12. doi: 10.1186/s12943-014-0276-y. - DOI - PMC - PubMed
-
- Weng M, Gong W, Ma M, Chu B, Qin Y, Zhang M, Lun X, McFadden G, Forsyth P, Yang Y, Quan Z. Targeting gallbladder cancer: oncolytic virotherapy with myxoma virus is enhanced by rapamycin in vitro and further improved by hyaluronan in vivo. Mol Cancer. 2014;13:82. doi: 10.1186/1476-4598-13-82. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

