Costs associated with failure to respond to treatment among patients with rheumatoid arthritis initiating TNFi therapy: a retrospective claims analysis
- PMID: 28506320
- PMCID: PMC5433023
- DOI: 10.1186/s13075-017-1293-1
Costs associated with failure to respond to treatment among patients with rheumatoid arthritis initiating TNFi therapy: a retrospective claims analysis
Abstract
Background: Tumor necrosis factor inhibitors (TNFi) are common second-line treatments for rheumatoid arthritis (RA). This study was designed to compare the real-world clinical and economic outcomes between patients with RA who responded to TNFi therapy and those who did not.
Methods: For this retrospective cohort analysis we used medical and pharmacy claims from members of 14 large U.S. commercial health plans represented in the HealthCore Integrated Research Database. Adult patients (aged ≥18 years) diagnosed with RA and initiating TNFi therapy (index date) between 1 January 2007 and 30 April 2014 were included in the study. Treatment response was assessed using a previously developed and validated claims-based algorithm. Patients classified as treatment responders in the 12 months postindex were matched 1:1 to nonresponders on important baseline characteristics, including sex, age, index TNFi agent, and comorbidities. The matched cohorts were then compared on their all-cause and RA-related healthcare resource use, and costs were assessed from a payer perspective during the first, second, and third years postindex using parametric tests, regressions, and a nonparametric bootstrap.
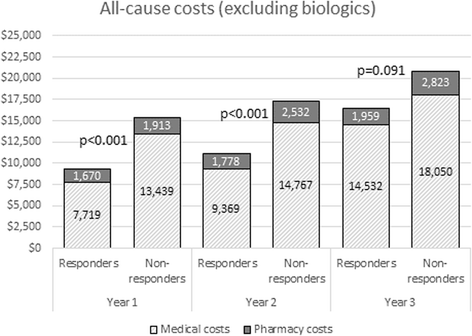
Results: A total of 7797 patients met the study inclusion criteria, among whom 2337 (30%) were classified as treatment responders. The responders had significantly lower all-cause hospitalizations, emergency department visits, and physical/occupational therapy visits than matched nonresponders during the first-year postindex. Mean total all-cause medical costs were $5737 higher for matched nonresponders, largely driven by outpatient visits and hospitalizations. Mean all-cause pharmacy costs (excluding costs of biologics) were $354 higher for matched nonresponders. Mean RA-related pharmacy costs (conventional synthetic and biologic drugs), however, were $8579 higher in the responder cohort, driven by higher adherence to their index TNFi agent (p < 0.01 for all comparisons). A similar pattern of cost differentiation was observed over years 2 and 3 of follow-up.
Conclusions: In this real-world study we found that, compared with matched nonresponders, patients who responded to TNFi treatments had lower all-cause medical, pharmacy, and total costs (excluding biologics) up to 3 years from initiation of TNFi therapy. These cost differences between the two cohorts provide a considerable offset to the cost of RA medications and should encourage close monitoring of treatment response to minimize disease progression with appropriate therapy choices.
Keywords: Biologic; Healthcare costs; Healthcare resource use; Real-world observational study; Rheumatoid arthritis; Treatment response.
Figures


References
-
- Kvien TK. Epidemiology and burden of illness of rheumatoid arthritis. Pharmacoeconomics. 2004;22(2 Suppl 1):1–12. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

