Prion-like Spreading in Tauopathies
- PMID: 28506438
- PMCID: PMC5640465
- DOI: 10.1016/j.biopsych.2017.04.003
Prion-like Spreading in Tauopathies
Abstract
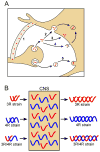
Tau is a microtubule-associated protein that functions in regulating cytoskeleton dynamics, especially in neurons. Misfolded and aggregated forms of tau produce pathological structures in a number of neurodegenerative diseases, including Alzheimer's disease (AD) and tauopathy dementias. These disorders can present with a sporadic etiology, such as in AD, or a familial etiology, such as in some cases of frontotemporal dementia with parkinsonism. Notably, the pathological features of tau pathology in these diseases can be very distinct. For example, the tau pathology in corticobasal degeneration is distinct from that of an AD patient. A wealth of evidence has emerged within the last decade to suggest that the misfolded tau in tauopathies possesses prion-like features and that such features may explain the diverse characteristics of tauopathies. The prion-like concept for tauopathies arose initially from the observation that the progressive accumulation of tau pathology as the symptoms of AD progress seemed to follow anatomically linked pathways. Subsequent studies in cell and animal models revealed that misfolded tau can propagate from cell to cell and from region to region in the brain through direct neuroanatomical connections. Studies in these cell and mouse models have demonstrated that experimentally propagated forms of misfolded tau can exist as conformationally distinct "strains" with unique biochemical, morphological, and neuropathological characteristics. This review discusses the clinical, pathological, and genetic diversity of tauopathies and the discoveries underlying the emerging view that the unique features of clinically distinct tauopathies may be a reflection of the strain of misfolded tau that propagates in each disease.
Keywords: Alzheimer’s disease; Animal models; Prion; Strains; Tau; Transmission.
Copyright © 2017 Society of Biological Psychiatry. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Wang Y, Mandelkow E. Tau in physiology and pathology. Nat Rev Neurosci. 2016;17:5–21. - PubMed
-
- Iqbal K, Liu F, Gong CX. Tau and neurodegenerative disease: the story so far. Nat Rev Neurol. 2016;12:15–27. - PubMed
-
- Lee VM-Y, Goedert M, Trojanoswki JQ. Neurodegenerative tauopathies. Annu Rev Neurosci. 2001;24:1121–1159. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

