MK2 Phosphorylates RIPK1 to Prevent TNF-Induced Cell Death
- PMID: 28506461
- PMCID: PMC5459754
- DOI: 10.1016/j.molcel.2017.05.003
MK2 Phosphorylates RIPK1 to Prevent TNF-Induced Cell Death
Abstract
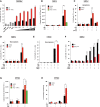
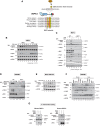
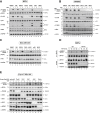
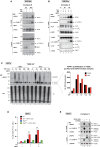
TNF is an inflammatory cytokine that upon binding to its receptor, TNFR1, can drive cytokine production, cell survival, or cell death. TNFR1 stimulation causes activation of NF-κB, p38α, and its downstream effector kinase MK2, thereby promoting transcription, mRNA stabilization, and translation of target genes. Here we show that TNF-induced activation of MK2 results in global RIPK1 phosphorylation. MK2 directly phosphorylates RIPK1 at residue S321, which inhibits its ability to bind FADD/caspase-8 and induce RIPK1-kinase-dependent apoptosis and necroptosis. Consistently, a phospho-mimetic S321D RIPK1 mutation limits TNF-induced death. Mechanistically, we find that phosphorylation of S321 inhibits RIPK1 kinase activation. We further show that cytosolic RIPK1 contributes to complex-II-mediated cell death, independent of its recruitment to complex-I, suggesting that complex-II originates from both RIPK1 in complex-I and cytosolic RIPK1. Thus, MK2-mediated phosphorylation of RIPK1 serves as a checkpoint within the TNF signaling pathway that integrates cell survival and cytokine production.
Keywords: IAPs; MK2; RIPK1; TNF; caspase-8; cell death; complex-II; cytokine; necroptosis; p38.
Copyright © 2017 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Balkwill F. Tumour necrosis factor and cancer. Nat. Rev. Cancer. 2009;9:361–371. - PubMed
-
- Berger S.B., Kasparcova V., Hoffman S., Swift B., Dare L., Schaeffer M., Capriotti C., Cook M., Finger J., Hughes-Earle A. Cutting edge: RIP1 kinase activity is dispensable for normal development but is a key regulator of inflammation in SHARPIN-deficient mice. J. Immunol. 2014;192:5476–5480. - PMC - PubMed
-
- Bertrand M.J., Milutinovic S., Dickson K.M., Ho W.C., Boudreault A., Durkin J., Gillard J.W., Jaquith J.B., Morris S.J., Barker P.A. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol. Cell. 2008;30:689–700. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

