Recruited Monocytes and Type 2 Immunity Promote Lung Regeneration following Pneumonectomy
- PMID: 28506464
- PMCID: PMC5501755
- DOI: 10.1016/j.stem.2017.03.024
Recruited Monocytes and Type 2 Immunity Promote Lung Regeneration following Pneumonectomy
Abstract
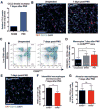
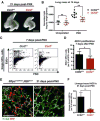
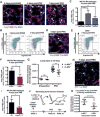
To investigate the role of immune cells in lung regeneration, we used a unilateral pneumonectomy model that promotes the formation of new alveoli in the remaining lobes. Immunofluorescence and single-cell RNA sequencing found CD115+ and CCR2+ monocytes and M2-like macrophages accumulating in the lung during the peak of type 2 alveolar epithelial stem cell (AEC2) proliferation. Genetic loss of function in mice and adoptive transfer studies revealed that bone marrow-derived macrophages (BMDMs) traffic to the lung through a CCL2-CCR2 chemokine axis and are required for optimal lung regeneration, along with Il4ra-expressing leukocytes. Our data suggest that these cells modulate AEC2 proliferation and differentiation. Finally, we provide evidence that group 2 innate lymphoid cells are a source of IL-13, which promotes lung regeneration. Together, our data highlight the potential for immunomodulatory therapies to stimulate alveologenesis in adults.
Keywords: ILC2; lung regeneration; macrophage; monocyte; pneumonectomy; type 2 alveolar pneumocyte.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures



Comment in
-
The puzzling mechanism of compensatory lung growth.Stem Cell Investig. 2018 Mar 31;5:8. doi: 10.21037/sci.2018.03.01. eCollection 2018. Stem Cell Investig. 2018. PMID: 29682515 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

