Autonomic dysreflexia after spinal cord injury: Systemic pathophysiology and methods of management
- PMID: 28506502
- PMCID: PMC5677594
- DOI: 10.1016/j.autneu.2017.05.002
Autonomic dysreflexia after spinal cord injury: Systemic pathophysiology and methods of management
Abstract
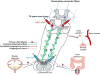
Traumatic spinal cord injury (SCI) has widespread physiological effects beyond the disruption of sensory and motor function, notably the loss of normal autonomic and cardiovascular control. Injury at or above the sixth thoracic spinal cord segment segregates critical spinal sympathetic neurons from supraspinal modulation which can result in a syndrome known as autonomic dysreflexia (AD). AD is defined as episodic hypertension and concomitant baroreflex-mediated bradycardia initiated by unmodulated sympathetic reflexes in the decentralized cord. This condition is often triggered by noxious yet unperceived visceral or somatic stimuli below the injury level and if severe enough can require immediate medical attention. Herein, we review the pathophysiological mechanisms germane to the development of AD, including maladaptive plasticity of neural circuits mediating abnormal sympathetic reflexes and hypersensitization of peripheral vasculature that collectively contribute to abnormal hemodynamics after SCI. Further, we discuss the systemic effects of recurrent AD and pharmacological treatments used to manage such episodes. Contemporary research avenues are then presented to better understand the relative contributions of underlying mechanisms and to elucidate the effects of recurring AD on cardiovascular and immune functions for developing more targeted and effective treatments to attenuate the development of this insidious syndrome following high-level SCI.
Keywords: Hypertension; Maladaptive plasticity; Primary afferent; Propriospinal; Sprouting; Sympathetic.
Copyright © 2017 Elsevier B.V. All rights reserved.
Conflict of interest statement
Figures
References
-
- Ackery AD, Norenberg MD, Krassioukov A. Calcitonin gene-related peptide immunoreactivity in chronic human spinal cord injury. Spinal cord. 2007;45:678–686. - PubMed
-
- Al Dera H, Habgood MD, Furness JB, Brock JA. Prominent contribution of L-type Ca2+ channels to cutaneous neurovascular transmission that is revealed after spinal cord injury augments vasoconstriction. Am J Physiol Heart Circ Physiol. 2012;302:H752–762. - PubMed
-
- Al-Chaer ED, Lawand NB, Westlund KN, Willis WD. Pelvic visceral input into the nucleus gracilis is largely mediated by the postsynaptic dorsal column pathway. J Neurophysiol. 1996;76:2675–2690. - PubMed
-
- Alan N, Ramer LM, Inskip JA, Golbidi S, Ramer MS, Laher I, Krassioukov AV. Recurrent autonomic dysreflexia exacerbates vascular dysfunction after spinal cord injury. Spine J. 2010;10:1108–1117. - PubMed
-
- Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. Journal of neurotrauma. 2004;21:1371–1383. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

