Advances in medical imaging for the diagnosis and management of common genitourinary cancers
- PMID: 28506596
- PMCID: PMC5931389
- DOI: 10.1016/j.urolonc.2017.04.014
Advances in medical imaging for the diagnosis and management of common genitourinary cancers
Abstract
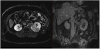
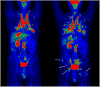
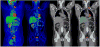
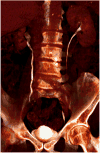
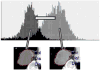
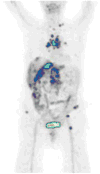
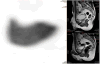
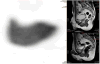
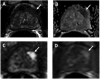
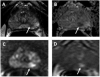
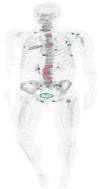
Medical imaging of the 3 most common genitourinary (GU) cancers-prostate adenocarcinoma, renal cell carcinoma, and urothelial carcinoma of the bladder-has evolved significantly during the last decades. The most commonly used imaging modalities for the diagnosis, staging, and follow-up of GU cancers are computed tomography, magnetic resonance imaging (MRI), and positron emission tomography (PET). Multiplanar multidetector computed tomography and multiparametric MRI with diffusion-weighted imaging are the main imaging modalities for renal cell carcinoma and urothelial carcinoma, and although multiparametric MRI is rapidly becoming the main imaging tool in the evaluation of prostate adenocarcinoma, biopsy is still required for diagnosis. Functional and molecular imaging using 18-fluorodeoxyglucose-PET and sodium fluoride-PET are essential for the diagnosis, and especially follow-up, of metastatic GU tumors. This review provides an overview of the latest advances in the imaging of these 3 major GU cancers.
Keywords: Bladder urothelial carcinoma; CT; MRI; PET; Prostate adenocarcinoma; Renal cell carcinoma.
Published by Elsevier Inc.
Figures


References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. - PubMed
-
- Cancer Facts & Figures 2016. Atlanta, GA: American Cancer Society; 2016.
-
- Ljungberg B, Bensalah K, Canfield S, Dabestani S, Hofmann F, Hora M, et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol. 2015;67:913–24. - PubMed
-
- Mueller-Lisse UG, Mueller-Lisse UL. Imaging of advanced renal cell carcinoma. World J Urol. 2010;28:253–61. - PubMed
-
- Rais-Bahrami S, Pietryga JA, Nix JW. Contemporary role of advanced imaging for bladder cancer staging. Urologic oncology. 2016;34:124–33. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

