Cell Biology of Tight Junction Barrier Regulation and Mucosal Disease
- PMID: 28507021
- PMCID: PMC5749156
- DOI: 10.1101/cshperspect.a029314
Cell Biology of Tight Junction Barrier Regulation and Mucosal Disease
Abstract
Mucosal surfaces are lined by epithelial cells. In the intestine, the epithelium establishes a selectively permeable barrier that supports nutrient absorption and waste secretion while preventing intrusion by luminal materials. Intestinal epithelia therefore play a central role in regulating interactions between the mucosal immune system and luminal contents, which include dietary antigens, a diverse intestinal microbiome, and pathogens. The paracellular space is sealed by the tight junction, which is maintained by a complex network of protein interactions. Tight junction dysfunction has been linked to a variety of local and systemic diseases. Two molecularly and biophysically distinct pathways across the intestinal tight junction are selectively and differentially regulated by inflammatory stimuli. This review discusses the mechanisms underlying these events, their impact on disease, and the potential of using these as paradigms for development of tight junction-targeted therapeutic interventions.
Copyright © 2018 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures



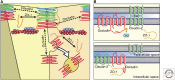
References
-
- Amasheh S, Meiri N, Gitter AH, Schoneberg T, Mankertz J, Schulzke JD, Fromm M. 2002. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J Cell Sci 115: 4969–4976. - PubMed
-
- Amasheh S, Fromm M, Gunzel D. 2011. Claudins of intestine and nephron—a correlation of molecular tight junction structure and barrier function. Acta Physiol (Oxf) 201: 133–140. - PubMed
-
- Angelow S, Schneeberger EE, Yu AS. 2007. Claudin-8 expression in renal epithelial cells augments the paracellular barrier by replacing endogenous claudin-2. J Membr Biol 215: 147–159. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
