The FOXO3/PGC-1β signaling axis is essential for cancer stem cell properties of pancreatic ductal adenocarcinoma
- PMID: 28507102
- PMCID: PMC5491768
- DOI: 10.1074/jbc.M116.772111
The FOXO3/PGC-1β signaling axis is essential for cancer stem cell properties of pancreatic ductal adenocarcinoma
Abstract
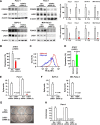
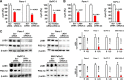
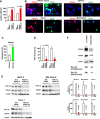
In 95% of patients with pancreatic ductal adenocarcinoma, recurrence is observed following chemotherapy. Findings from several studies have indicated that cancer stem cells (CSCs) are resistant to anticancer agents and may be involved in cancer recurrence and metastasis. The CD44 protein is a major CSC marker, and CD44 also plays an indispensable role in the CSC properties in several cancers, including pancreatic cancer; however, no clinical approach exists to inhibit CD44 activity. Here, we have performed knock-in/knockdown experiments, and we demonstrate that the forkhead box O3 (FOXO3)/liver kinase B1 (LKB1)/AMP-activated protein kinase/peroxisome proliferator-activated receptor-γ co-activator-1β (PGC-1β)/pyruvate dehydrogenase-A1 pathway is essential for CD44 expression and CSC properties. We observed that patients exhibiting high pyruvate dehydrogenase-A1 expression have a poor prognosis. Systemic PGC-1β knock-out mice are fertile and viable and do not exhibit an overt phenotype under normal conditions. This suggests that cGMP induction and PGC-1β inhibition represent potential strategies for treating patients with pancreatic ductal adenocarcinoma.
Keywords: CD44; FOXO; PGC-1β; liver kinase B1 (LKB1); pancreatic cancer; stem cells.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures




Similar articles
-
FOXO3 is essential for CD44 expression in pancreatic cancer cells.Oncogene. 2017 May 11;36(19):2643-2654. doi: 10.1038/onc.2016.426. Epub 2016 Nov 28. Oncogene. 2017. PMID: 27893718
-
Knockdown of FOXO3a induces epithelial-mesenchymal transition and promotes metastasis of pancreatic ductal adenocarcinoma by activation of the β-catenin/TCF4 pathway through SPRY2.J Exp Clin Cancer Res. 2019 Jan 28;38(1):38. doi: 10.1186/s13046-019-1046-x. J Exp Clin Cancer Res. 2019. Retraction in: J Exp Clin Cancer Res. 2022 Jun 17;41(1):204. doi: 10.1186/s13046-022-02413-2. PMID: 30691517 Free PMC article. Retracted.
-
Aspartate β-hydroxylase promotes pancreatic ductal adenocarcinoma metastasis through activation of SRC signaling pathway.J Hematol Oncol. 2019 Dec 30;12(1):144. doi: 10.1186/s13045-019-0837-z. J Hematol Oncol. 2019. PMID: 31888763 Free PMC article.
-
Role of cancer stem cells in pancreatic ductal adenocarcinoma.Nat Rev Clin Oncol. 2009 Oct;6(10):580-6. doi: 10.1038/nrclinonc.2009.127. Epub 2009 Aug 18. Nat Rev Clin Oncol. 2009. PMID: 19687789 Review.
-
Nestin and other putative cancer stem cell markers in pancreatic cancer.Med Mol Morphol. 2012 Jun;45(2):59-65. doi: 10.1007/s00795-012-0571-x. Epub 2012 Jun 21. Med Mol Morphol. 2012. PMID: 22718289 Review.
Cited by
-
High expression of cytoplasmic FOXO3 protein associated with poor prognosis of rectal cancer patients: A study from Swedish clinical trial of preoperative radiotherapy to big database analysis.Heliyon. 2023 Apr 10;9(5):e15342. doi: 10.1016/j.heliyon.2023.e15342. eCollection 2023 May. Heliyon. 2023. PMID: 37131452 Free PMC article.
-
Proliferation and Apoptosis of B-Cell Lymphoma Cells under Targeted Regulation of FOXO3 by miR-155.Mediterr J Hematol Infect Dis. 2020 Nov 1;12(1):e2020073. doi: 10.4084/MJHID.2020.073. eCollection 2020. Mediterr J Hematol Infect Dis. 2020. PMID: 33194147 Free PMC article.
-
Targeting the non-canonical AKT-FOXO3a axis: A potential therapeutic strategy for oral squamous cell carcinoma.EBioMedicine. 2019 Nov;49:6-8. doi: 10.1016/j.ebiom.2019.10.020. Epub 2019 Oct 21. EBioMedicine. 2019. PMID: 31648985 Free PMC article. No abstract available.
-
Differentially Expressed microRNAs in MIA PaCa-2 and PANC-1 Pancreas Ductal Adenocarcinoma Cell Lines are Involved in Cancer Stem Cell Regulation.Int J Mol Sci. 2019 Sep 10;20(18):4473. doi: 10.3390/ijms20184473. Int J Mol Sci. 2019. PMID: 31510100 Free PMC article.
-
Exploration of the System-Level Mechanisms of the Herbal Drug FDY003 for Pancreatic Cancer Treatment: A Network Pharmacological Investigation.Evid Based Complement Alternat Med. 2022 May 10;2022:7160209. doi: 10.1155/2022/7160209. eCollection 2022. Evid Based Complement Alternat Med. 2022. PMID: 35591866 Free PMC article.
References
-
- Siegel R. L., Miller K. D., and Jemal A. (2015) Cancer statistics, 2015. CA Cancer J. Clin. 65, 5–29 - PubMed
-
- Burris H. A. 3rd, Moore M. J., Andersen J., Green M. R., Rothenberg M. L., Modiano M. R., Cripps M. C., Portenoy R. K., Storniolo A. M., Tarassoff P., Nelson R., Dorr F. A., Stephens C. D., and Von Hoff D. D. (1997) Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J. Clin. Oncol. 15, 2403–2413 - PubMed
-
- Conroy T., Desseigne F., Ychou M., Bouché O., Guimbaud R., Bécouarn Y., Adenis A., Raoul J. L., Gourgou-Bourgade S., de la Fouchardière C., Bennouna J., Bachet J. B., Khemissa-Akouz F., Péré-Vergé D., Delbaldo C., et al. (2011) FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 364, 1817–1825 - PubMed
-
- Quint K., Tonigold M., Di Fazio P., Montalbano R., Lingelbach S., Rückert F., Alinger B., Ocker M., and Neureiter D. (2012) Pancreatic cancer cells surviving gemcitabine treatment express markers of stem cell differentiation and epithelial-mesenchymal transition. Int. J. Oncol. 41, 2093–2102 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

