In Vitro Cross-Resistance Profiles of Rilpivirine, Dapivirine, and MIV-150, Nonnucleoside Reverse Transcriptase Inhibitor Microbicides in Clinical Development for the Prevention of HIV-1 Infection
- PMID: 28507107
- PMCID: PMC5487620
- DOI: 10.1128/AAC.00277-17
In Vitro Cross-Resistance Profiles of Rilpivirine, Dapivirine, and MIV-150, Nonnucleoside Reverse Transcriptase Inhibitor Microbicides in Clinical Development for the Prevention of HIV-1 Infection
Abstract
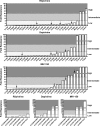
Rilpivirine (RPV), dapivirine (DPV), and MIV-150 are in development as microbicides. It is not known whether they will block infection of circulating nonnucleoside reverse transcriptase inhibitor (NNRTI)-resistant human immunodeficiency virus type 1 (HIV-1) variants. Here, we demonstrate that the activity of DPV and MIV-150 is compromised by many resistant viruses containing single or double substitutions. High DPV genital tract concentrations from DPV ring use may block replication of resistant viruses. However, MIV-150 genital tract concentrations may be insufficient to inhibit many resistant viruses, including those harboring K103N or Y181C.
Keywords: HIV-1; MIV-150; NNRTI; antiretroviral resistance; dapivirine; prevention.
Copyright © 2017 American Society for Microbiology.
Figures
Similar articles
-
Long-Acting Rilpivirine (RPV) Preexposure Prophylaxis Does Not Inhibit Vaginal Transmission of RPV-Resistant HIV-1 or Select for High-Frequency Drug Resistance in Humanized Mice.J Virol. 2020 Mar 31;94(8):e01912-19. doi: 10.1128/JVI.01912-19. Print 2020 Mar 31. J Virol. 2020. PMID: 31969438 Free PMC article.
-
Frequent Cross-Resistance to Dapivirine in HIV-1 Subtype C-Infected Individuals after First-Line Antiretroviral Therapy Failure in South Africa.Antimicrob Agents Chemother. 2017 Jan 24;61(2):e01805-16. doi: 10.1128/AAC.01805-16. Print 2017 Feb. Antimicrob Agents Chemother. 2017. PMID: 27895013 Free PMC article.
-
[Resistance profile of rilpivirine].Enferm Infecc Microbiol Clin. 2013 Jun;31 Suppl 2:36-43. doi: 10.1016/S0213-005X(13)70141-1. Enferm Infecc Microbiol Clin. 2013. PMID: 24252532 Review. Spanish.
-
Distinct resistance patterns to etravirine and rilpivirine in viruses containing nonnucleoside reverse transcriptase inhibitor mutations at baseline.AIDS. 2013 Mar 27;27(6):879-887. doi: 10.1097/QAD.0b013e32835d9f6d. AIDS. 2013. PMID: 23262501
-
Etravirine and rilpivirine: nonnucleoside reverse transcriptase inhibitors with activity against human immunodeficiency virus type 1 strains resistant to previous nonnucleoside agents.Pharmacotherapy. 2009 Mar;29(3):281-94. doi: 10.1592/phco.29.3.281. Pharmacotherapy. 2009. PMID: 19249947 Review.
Cited by
-
Conformational Changes in HIV-1 Reverse Transcriptase that Facilitate Its Maturation.Structure. 2019 Oct 1;27(10):1581-1593.e3. doi: 10.1016/j.str.2019.08.004. Epub 2019 Aug 27. Structure. 2019. PMID: 31471129 Free PMC article.
-
Discovery of Benzisothiazolone Derivatives as Bifunctional Inhibitors of HIV-1 Reverse Transcriptase DNA Polymerase and Ribonuclease H Activities.Biomolecules. 2024 Jul 9;14(7):819. doi: 10.3390/biom14070819. Biomolecules. 2024. PMID: 39062532 Free PMC article.
-
Mutations in the HIV-1 3'-Polypurine Tract and Integrase Strand Transfer Inhibitor Resistance.Antimicrob Agents Chemother. 2021 May 18;65(6):e02432-20. doi: 10.1128/AAC.02432-20. Print 2021 May 18. Antimicrob Agents Chemother. 2021. PMID: 33722887 Free PMC article. No abstract available.
-
Approved HIV reverse transcriptase inhibitors in the past decade.Acta Pharm Sin B. 2022 Apr;12(4):1567-1590. doi: 10.1016/j.apsb.2021.11.009. Epub 2021 Nov 16. Acta Pharm Sin B. 2022. PMID: 35847492 Free PMC article. Review.
-
Indolylarylsulfones, a fascinating story of highly potent human immunodeficiency virus type 1 non-nucleoside reverse transcriptase inhibitors.Antivir Chem Chemother. 2018 Jan-Dec;26:2040206617753443. doi: 10.1177/2040206617753443. Antivir Chem Chemother. 2018. PMID: 29417826 Free PMC article. Review.
References
-
- Baeten JM, Palanee-Phillips T, Brown ER, Schwartz K, Soto-Torres LE, Govender V, Mgodi NM, Matovu Kiweewa F, Nair G, Mhlanga F, Siva S, Bekker LG, Jeenarain N, Gaffoor Z, Martinson F, Makanani B, Pather A, Naidoo L, Husnik M, Richardson BA, Parikh UM, Mellors JW, Marzinke MA, Hendrix CW, van der Straten A, Ramjee G, Chirenje ZM, Nakabiito C, Taha TE, Jones J, Mayo A, Scheckter R, Berthiaume J, Livant E, Jacobson C, Ndase P, White R, Patterson K, Germuga D, Galaska B, Bunge K, Singh D, Szydlo DW, Montgomery ET, Mensch BS, Torjesen K, Grossman CI, Chakhtoura N, Nel A, Rosenberg Z, McGowan I, Hillier S, MTN-020–ASPIRE Study Team. 2016. Use of a vaginal ring containing dapivirine for HIV-1 prevention in women. N Engl J Med 375:2121–2132. doi:10.1056/NEJMoa1506110. - DOI - PMC - PubMed
-
- Nel A, van Niekerk N, Kapiga S, Bekker LG, Gama C, Gill K, Kamali A, Kotze P, Louw C, Mabude Z, Miti N, Kusemererwa S, Tempelman H, Carstens H, Devlin B, Isaacs M, Malherbe M, Mans W, Nuttall J, Russell M, Ntshele S, Smit M, Solai L, Spence P, Steytler J, Windle K, Borremans M, Resseler S, Van Roey J, Parys W, Vangeneugden T, Van Baelen B, Rosenberg Z, Ring Study Team. 2016. Safety and efficacy of a dapivirine vaginal ring for HIV prevention in women. N Engl J Med 375:2133–2143. doi:10.1056/NEJMoa1602046. - DOI - PubMed
-
- Friedland BA, Hoesley CJ, Plagianos M, Hoskin E, Zhang S, Teleshova N, Alami M, Novak L, Kleinbeck KR, Katzen LL, Zydowsky TM, Fernández-Romero JA, Creasy GW. 2016. First-in-human trial of MIV-150 and zinc acetate coformulated in a carrageenan gel: safety, pharmacokinetics, acceptability, adherence, and pharmacodynamics. J Acquir Immune Defic Syndr 73:489–496. doi:10.1097/QAI.0000000000001136. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

