Efficacy of a Binuclear Cyclopalladated Compound Therapy for Cutaneous Leishmaniasis in the Murine Model of Infection with Leishmania amazonensis and Its Inhibitory Effect on Topoisomerase 1B
- PMID: 28507113
- PMCID: PMC5527659
- DOI: 10.1128/AAC.00688-17
Efficacy of a Binuclear Cyclopalladated Compound Therapy for Cutaneous Leishmaniasis in the Murine Model of Infection with Leishmania amazonensis and Its Inhibitory Effect on Topoisomerase 1B
Abstract
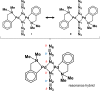
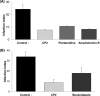
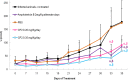
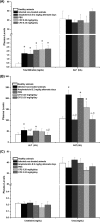
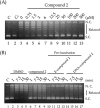
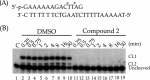
Leishmaniasis is a disease found throughout the (sub)tropical parts of the world caused by protozoan parasites of the Leishmania genus. Despite the numerous problems associated with existing treatments, pharmaceutical companies continue to neglect the development of better ones. The high toxicity of current drugs combined with emerging resistance makes the discovery of new therapeutic alternatives urgent. We report here the evaluation of a binuclear cyclopalladated complex containing Pd(II) and N,N'-dimethylbenzylamine (Hdmba) against Leishmania amazonensis The compound [Pd(dmba)(μ-N3)]2 (CP2) inhibits promastigote growth (50% inhibitory concentration [IC50] = 13.2 ± 0.7 μM) and decreases the proliferation of intracellular amastigotes in in vitro incubated macrophages (IC50 = 10.2 ± 2.2 μM) without a cytotoxic effect when tested against peritoneal macrophages (50% cytotoxic concentration = 506.0 ± 10.7 μM). In addition, CP2 was also active against T. cruzi intracellular amastigotes (IC50 = 2.3 ± 0.5 μM, selective index = 225), an indication of its potential for use in Chagas disease therapy. In vivo assays using L. amazonensis-infected BALB/c showed an 80% reduction in parasite load compared to infected and nontreated animals. Also, compared to amphotericin B treatment, CP2 did not show any side effects, which was corroborated by the analysis of plasma levels of different hepatic and renal biomarkers. Furthermore, CP2 was able to inhibit Leishmania donovani topoisomerase 1B (Ldtopo1B), a potentially important target in this parasite. (This study has been registered at ClinicalTrials.gov under identifier NCT02169141.).
Keywords: Chagas disease; Leishmania amazonensis; Leishmania donovani; Trypanosoma cruzi; cyclopalladated complex; leishmaniasis; topoisomerase 1B.
Copyright © 2017 Velásquez et al.
Figures

References
-
- Torres FA, Passalacqua TG, Velásquez AM, de Souza RA, Colepicolo P, Graminha MA. 2014. New drugs with antiprotozoal activity from marine algae: a review. Braz J Pharmacogn 24:265–276. doi:10.1016/j.bjp.2014.07.001. - DOI
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

