Highly active catalyst derived from a 3D foam of Fe(PO3)2/Ni2P for extremely efficient water oxidation
- PMID: 28507120
- PMCID: PMC5465920
- DOI: 10.1073/pnas.1701562114
Highly active catalyst derived from a 3D foam of Fe(PO3)2/Ni2P for extremely efficient water oxidation
Abstract
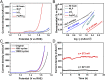
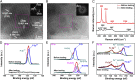
Commercial hydrogen production by electrocatalytic water splitting will benefit from the realization of more efficient and less expensive catalysts compared with noble metal catalysts, especially for the oxygen evolution reaction, which requires a current density of 500 mA/cm2 at an overpotential below 300 mV with long-term stability. Here we report a robust oxygen-evolving electrocatalyst consisting of ferrous metaphosphate on self-supported conductive nickel foam that is commercially available in large scale. We find that this catalyst, which may be associated with the in situ generated nickel-iron oxide/hydroxide and iron oxyhydroxide catalysts at the surface, yields current densities of 10 mA/cm2 at an overpotential of 177 mV, 500 mA/cm2 at only 265 mV, and 1,705 mA/cm2 at 300 mV, with high durability in alkaline electrolyte of 1 M KOH even after 10,000 cycles, representing activity enhancement by a factor of 49 in boosting water oxidation at 300 mV relative to the state-of-the-art IrO2 catalyst.
Keywords: commercial utilization; electrocatalytic water splitting; ferrous metaphosphate; iron; oxygen evolution reaction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Dresselhaus MS, Thomas IL. Alternative energy technologies. Nature. 2001;414:332–337. - PubMed
-
- Gray HB. Powering the planet with solar fuel. Nat Chem. 2009;1:7. - PubMed
-
- Walter MG, et al. Solar water splitting cells. Chem Rev. 2010;110:6446–6473. - PubMed
-
- Gao S, et al. Partially oxidized atomic cobalt layers for carbon dioxide electroreduction to liquid fuel. Nature. 2016;529:68–71. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

