Control of metastatic niche formation by targeting APBA3/Mint3 in inflammatory monocytes
- PMID: 28507122
- PMCID: PMC5465932
- DOI: 10.1073/pnas.1703171114
Control of metastatic niche formation by targeting APBA3/Mint3 in inflammatory monocytes
Abstract
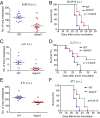
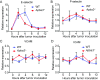
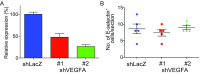
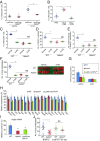
Cancer metastasis is intricately orchestrated by both cancer and normal cells, such as endothelial cells and macrophages. Monocytes/macrophages, which are often co-opted by cancer cells and promote tumor malignancy, acquire more than half of their energy from glycolysis even during normoxic conditions. This glycolytic activity is maintained during normoxia by the functions of hypoxia inducible factor 1 (HIF-1) and its activator APBA3. The mechanism by which APBA3 inhibition partially suppresses macrophage function and affects cancer metastasis is of interest in view of avoidance of the adverse effects of complete suppression of macrophage function during therapy. Here, we report that APBA3-deficient mice show reduced metastasis, with no apparent effect on primary tumor growth. APBA3 deficiency in inflammatory monocytes, which strongly express the chemokine receptor CCR2 and are recruited toward chemokine CCL2 from metastatic sites, hampers glycolysis-dependent chemotaxis of cells toward metastatic sites and inhibits VEGFA expression, similar to the effects observed with HIF-1 deficiency. Host APBA3 induces VEGFA-mediated E-selectin expression in the endothelial cells of target organs, thereby promoting extravasation of cancer cells and micrometastasis formation. Administration of E-selectin-neutralizing antibody also abolished host APBA3-mediated metastatic formation. Thus, targeting APBA3 is useful for controlling metastatic niche formation by inflammatory monocytes.
Keywords: APBA3/Mint3; macrophage; metastasis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

