Interleukin-1 and TRAF6-dependent activation of TAK1 in the absence of TAB2 and TAB3
- PMID: 28507161
- PMCID: PMC5632801
- DOI: 10.1042/BCJ20170288
Interleukin-1 and TRAF6-dependent activation of TAK1 in the absence of TAB2 and TAB3
Abstract
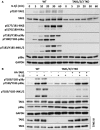
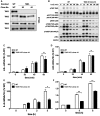
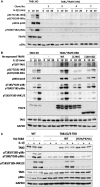
Interleukin-1 (IL-1) signaling induces the formation of Lys63-linked ubiquitin (K63-Ub) chains, which are thought to activate the 'master' protein kinase TGFβ-activated kinase 1 (TAK1) by interacting with its TAK1-binding 2 (TAB2) and TAB3 subunits. Here, we report that IL-1β can also activate the TAB1-TAK1 heterodimer present in TAB2/TAB3 double knockout (DKO) IL-1 receptor-expressing cells. The IL-1β-dependent activation of the TAB1-TAK1 heterodimer in TAB2/3 DKO cells is required for the expression and E3 ligase activity of tumor necrosis factor receptor-associated factor 6 (TRAF6) and is reduced by the small interfering RNA (siRNA) knockdown of ubiquitin conjugating 13 (Ubc13), an E2-conjugating enzyme that directs the formation of K63-Ub chains. IL-1β signaling was restored to TAB1/2/3 triple KO cells by the re-expression of either TAB1 or TAB2, but not by an ubiquitin binding-defective mutant of TAB2. We conclude that IL-1β can induce the activation of TAK1 in two ways, only one of which requires the binding of K63-Ub chains to TAB2/3. The early IL-1β-stimulated, TAK1-dependent activation of p38α mitogen-activated protein (MAP) kinase and the canonical IκB kinase (IKK) complex, as well as the NF-κB-dependent transcription of immediate early genes, was similar in TAB2/3 DKO cells and TAB2/3-expressing cells. However, in contrast with TAB2/3-expressing cells, IL-1β signaling was transient in TAB2/3 DKO cells, and the activation of c-Jun N-terminal kinase 1 (JNK1), JNK2 and p38γ was greatly reduced at all times. These observations indicate a role for TAB2/3 in directing the TAK1-dependent activation of MAP kinase kinases that switch on JNK1/2 and p38γ MAP kinases. These observations and the transient activation of the TAB1-TAK1 heterodimer may explain why IL-1β-dependent IL-8 mRNA formation was abolished in TAB2/3 DKO cells.
Keywords: TAB1; TAB2; TAK1; TRAF6; interleukin-1; ubiquitin.
© 2017 The Author(s).
Conflict of interest statement
The Authors declare that there are no competing interests associated with the manuscript information.
Figures



Similar articles
-
Roles for TAB1 in regulating the IL-1-dependent phosphorylation of the TAB3 regulatory subunit and activity of the TAK1 complex.Biochem J. 2008 Feb 1;409(3):711-22. doi: 10.1042/BJ20071149. Biochem J. 2008. PMID: 18021073
-
TAK1-dependent signaling requires functional interaction with TAB2/TAB3.J Biol Chem. 2007 Feb 9;282(6):3918-28. doi: 10.1074/jbc.M608867200. Epub 2006 Dec 8. J Biol Chem. 2007. PMID: 17158449 Free PMC article.
-
TAB2 and TAB3 activate the NF-kappaB pathway through binding to polyubiquitin chains.Mol Cell. 2004 Aug 27;15(4):535-48. doi: 10.1016/j.molcel.2004.08.008. Mol Cell. 2004. PMID: 15327770
-
The role of hybrid ubiquitin chains in the MyD88 and other innate immune signalling pathways.Cell Death Differ. 2017 Jul;24(7):1153-1159. doi: 10.1038/cdd.2017.17. Epub 2017 May 5. Cell Death Differ. 2017. PMID: 28475177 Free PMC article. Review.
-
Post-Translational Modifications of the TAK1-TAB Complex.Int J Mol Sci. 2017 Jan 19;18(1):205. doi: 10.3390/ijms18010205. Int J Mol Sci. 2017. PMID: 28106845 Free PMC article. Review.
Cited by
-
Influence of Type 2 Diabetes and Adipose Tissue Dysfunction on Breast Cancer and Potential Benefits from Nutraceuticals Inducible in Microalgae.Nutrients. 2024 Sep 25;16(19):3243. doi: 10.3390/nu16193243. Nutrients. 2024. PMID: 39408212 Free PMC article. Review.
-
Inhibition of NFAM1 suppresses phospho-SAPK/JNK signaling during osteoclast differentiation and bone resorption.J Cell Biochem. 2021 Oct;122(10):1534-1543. doi: 10.1002/jcb.30076. Epub 2021 Jul 6. J Cell Biochem. 2021. PMID: 34228377 Free PMC article.
-
TAK1-TABs Complex: A Central Signalosome in Inflammatory Responses.Front Immunol. 2021 Jan 5;11:608976. doi: 10.3389/fimmu.2020.608976. eCollection 2020. Front Immunol. 2021. PMID: 33469458 Free PMC article. Review.
-
Arginyltransferase knockdown attenuates cardiac hypertrophy and fibrosis through TAK1-JNK1/2 pathway.Sci Rep. 2020 Jan 17;10(1):598. doi: 10.1038/s41598-019-57379-7. Sci Rep. 2020. PMID: 31953451 Free PMC article.
-
Bibliometric and visualized analysis on global trends and hotspots of TAK1 in regulated cell death: 1999 to 2024.Front Immunol. 2024 Oct 15;15:1437570. doi: 10.3389/fimmu.2024.1437570. eCollection 2024. Front Immunol. 2024. PMID: 39474417 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

