Matrix stiffness regulates migration of human lung fibroblasts
- PMID: 28507166
- PMCID: PMC5430127
- DOI: 10.14814/phy2.13281
Matrix stiffness regulates migration of human lung fibroblasts
Abstract
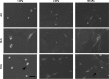
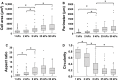
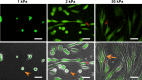
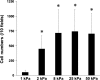
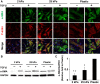
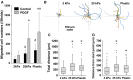
In patients with pulmonary diseases such as idiopathic pulmonary fibrosis and severe acute respiratory distress syndrome, progressive pulmonary fibrosis is caused by dysregulated wound healing via activation of fibroblasts after lung inflammation or severe damage. Migration of fibroblasts toward the fibrotic lesions plays an important role in pulmonary fibrosis. Fibrotic tissue in the lung is much stiffer than normal lung tissue. Emerging evidence supports the hypothesis that the stiffness of the matrix is not only a consequence of fibrosis, but also can induce fibroblast activation. Nevertheless, the effects of substrate rigidity on migration of lung fibroblasts have not been fully elucidated. We evaluated the effects of substrate stiffness on the morphology, α-smooth muscle actin (α-SMA) expression, and cell migration of primary human lung fibroblasts by using polyacrylamide hydrogels with stiffnesses ranging from 1 to 50 kPa. Cell motility was assessed by platelet-derived growth factor (PDGF)-induced chemotaxis and random walk migration assays. As the stiffness of substrates increased, fibroblasts became spindle-shaped and spread. Expression of α-SMA proteins was higher on the stiffer substrates (25 kPa gel and plastic dishes) than on the soft 2 kPa gel. Both PDGF-induced chemotaxis and random walk migration of fibroblasts precultured on stiff substrates (25 kPa gel and plastic dishes) were significantly higher than those of cells precultured on 2 kPa gel. Transfection of the fibroblasts with short interfering RNA for α-SMA inhibited cell migration. These findings suggest that fibroblast activation induced by a stiff matrix is involved in mechanisms of the pathophysiology of pulmonary fibrosis.
Keywords: Matrix stiffness; mechanotransduction; migration; pulmonary fibrosis; α‐smooth muscle actin.
© 2017 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of The Physiological Society and the American Physiological Society.
Figures

References
-
- Aso, H. , Ito S., Mori A., Suganuma N., Morioka M., Takahara N., et al. 2013. Differential regulation of airway smooth muscle cell migration by E‐prostanoid receptor subtypes. Am. J. Respir. Cell Mol. Biol. 48:322–329. - PubMed
-
- Balestrini, J. L. , Chaudhry S., Sarrazy V., Koehler A., and Hinz B.. 2012. The mechanical memory of lung myofibroblasts. Integr. Biol. (Camb) 4:410–421. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

