PINK1/Parkin-Dependent Mitochondrial Surveillance: From Pleiotropy to Parkinson's Disease
- PMID: 28507507
- PMCID: PMC5410576
- DOI: 10.3389/fnmol.2017.00120
PINK1/Parkin-Dependent Mitochondrial Surveillance: From Pleiotropy to Parkinson's Disease
Abstract
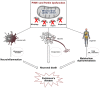
Parkinson's disease (PD) is one of the most frequent neurodegenerative disease caused by the preferential, progressive degeneration of the dopaminergic (DA) neurons of the substantia nigra (SN) pars compacta. PD is characterized by a multifaceted pathological process involving protein misfolding, mitochondrial dysfunction, neuroinflammation and metabolism deregulation. The molecular mechanisms governing the complex interplay between the different facets of this process are still unknown. PARK2/Parkin and PARK6/PINK1, two genes responsible for familial forms of PD, act as a ubiquitous core signaling pathway, coupling mitochondrial stress to mitochondrial surveillance, by regulating mitochondrial dynamics, the removal of damaged mitochondrial components by mitochondria-derived vesicles, mitophagy, and mitochondrial biogenesis. Over the last decade, PINK1/Parkin-dependent mitochondrial quality control emerged as a pleiotropic regulatory pathway. Loss of its function impinges on a number of physiological processes suspected to contribute to PD pathogenesis. Its role in the regulation of innate immunity and inflammatory processes stands out, providing compelling support to the contribution of non-cell-autonomous immune mechanisms in PD. In this review, we illustrate the central role of this multifunctional pathway at the crossroads between mitochondrial stress, neuroinflammation and metabolism. We discuss how its dysfunction may contribute to PD pathogenesis and pinpoint major unresolved questions in the field.
Keywords: Parkinson disease; Pink1/Parkin; UPRmt; mitochondrial quality control; mitochondrial stress; neuroinflammation; neuronal death.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

