[Ag115S34(SCH2C6H4t Bu)47(dpph)6]: synthesis, crystal structure and NMR investigations of a soluble silver chalcogenide nanocluster
- PMID: 28507679
- PMCID: PMC5408567
- DOI: 10.1039/c6sc04578b
[Ag115S34(SCH2C6H4t Bu)47(dpph)6]: synthesis, crystal structure and NMR investigations of a soluble silver chalcogenide nanocluster
Abstract
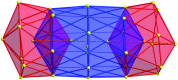
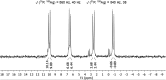
With the aim to synthesize soluble cluster molecules, the silver salt of (4-(tert-butyl)phenyl)methanethiol [AgSCH2C6H4t Bu] was applied as a suitable precursor for the formation of a nanoscale silver sulfide cluster. In the presence of 1,6-(diphenylphosphino)hexane (dpph), the 115 nuclear silver cluster [Ag115S34(SCH2C6H4t Bu)47(dpph)6] was obtained. The molecular structure of this compound was elucidated by single crystal X-ray analysis and fully characterized by spectroscopic techniques. In contrast to most of the previously published cluster compounds with more than a hundred heavy atoms, this nanoscale inorganic molecule is soluble in organic solvents, which allowed a comprehensive investigation in solution by UV-Vis spectroscopy and one- and two-dimensional NMR spectroscopy including 31P/109Ag-HSQC and DOSY experiments. These are the first heteronuclear NMR investigations on coinage metal chalcogenides. They give some first insight into the behavior of nanoscale silver sulfide clusters in solution. Additionally, molecular weight determinations were performed by 2D analytical ultracentrifugation and HR-TEM investigations confirm the presence of size-homogeneous nanoparticles present in solution.
Figures





References
-
- Fuhr O., Dehnen S., Fenske D. Chem. Soc. Rev. 2013;42:1871–1906. - PubMed
-
- Corrigan J. F., Fuhr O., Fenske D. Adv. Mater. 2009;21:1867–1871.
-
- Soloviev V. N., Eichhöfer A., Fenske D., Banin U. J. Am. Chem. Soc. 2001;123:2354–2364. - PubMed
-
- Yang X.-X., Issac I., Lebedkin S., Kuhn M., Weigend F., Fenske D., Fuhr O., Eichhofer A. Chem. Commun. 2014;50:11043–11045. - PubMed
-
- Eichhöfer A., Corrigan J. F., Fenske D., Tröster E. Z. Anorg. Allg. Chem. 2000;626:338–348.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

