Adaptive T cell responses induced by oncolytic Herpes Simplex Virus-granulocyte macrophage-colony-stimulating factor therapy expanded by dendritic cell and cytokine-induced killer cell adoptive therapy
- PMID: 28507788
- PMCID: PMC5414875
- DOI: 10.1080/2162402X.2016.1264563
Adaptive T cell responses induced by oncolytic Herpes Simplex Virus-granulocyte macrophage-colony-stimulating factor therapy expanded by dendritic cell and cytokine-induced killer cell adoptive therapy
Abstract
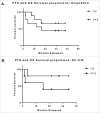
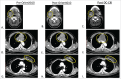
Purpose: Although local oncolytic viral therapy (OVT) may enhance tumor lysis, antigen release, and adaptive immune responses, systemic antitumor responses post-therapy are limited. Adoptive immunotherapy with autologous dendritic cells (DC) and cytokine-induced killer cells (DC-CIK) synergizes with systemic therapies. We hypothesized that OVT with Herpes Simplex Virus-granulocyte macrophage-colony-stimulating factor (HSV-GM-CSF) would induce adaptive T cell responses that could be expanded systemically with sequential DC-CIK therapy. Patients and Methods: We performed a pilot study of intratumoral HSV-GM-CSF OVT followed by autologous DC-CIK cell therapy. In addition to safety and clinical endpoints, we monitored adaptive T cell responses by quantifying T cell receptor (TCR) populations in pre-oncolytic therapy, post-oncolytic therapy, and after DC-CIK therapy. Results: Nine patients with advanced malignancy were treated with OVT (OrienX010), of whom seven experienced stable disease (SD). Five of the OVT treated patients underwent leukapheresis, generation, and delivery of DC-CIKs, and two had SD, whereas three progressed. T cell receptor sequencing of TCR β sequences one month after OVT therapy demonstrates a dynamic TCR repertoire in response to OVT therapy in the majority of patients with the systematic expansion of multiple T cell clone populations following DC-CIK therapy. This treatment was well tolerated and long-term event free and overall survival was observed in six of the nine patients. Conclusions: Strategies inducing the local activation of tumor-specific immune responses can be combined with adoptive cellular therapies to expand the adaptive T cell responses systemically and further studies are warranted.
Keywords: Dendritic cells; Herpes Simplex Virus; T cell receptor sequencing; oncolytic viral therapy.
Figures

Similar articles
-
[Therapy of relapsed or refractory non-Hodgkin's lymphoma by antigen specific dendritic cells-activated lymphocytes].Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2010 Feb;18(1):219-23. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2010. PMID: 20137151 Clinical Trial. Chinese.
-
Autologous Dendritic Cell-Cytokine Induced Killer Cell Immunotherapy Combined with S-1 Plus Cisplatin in Patients with Advanced Gastric Cancer: A Prospective Study.Clin Cancer Res. 2019 Mar 1;25(5):1494-1504. doi: 10.1158/1078-0432.CCR-18-2360. Epub 2018 Dec 4. Clin Cancer Res. 2019. PMID: 30514775
-
Optimized biodegradable polymeric reservoir-mediated local and sustained co-delivery of dendritic cells and oncolytic adenovirus co-expressing IL-12 and GM-CSF for cancer immunotherapy.J Control Release. 2017 Aug 10;259:115-127. doi: 10.1016/j.jconrel.2017.03.028. Epub 2017 Mar 20. J Control Release. 2017. PMID: 28336378
-
Herpes simplex virus oncolytic vaccine therapy in melanoma.Expert Opin Biol Ther. 2010 Jul;10(7):1145-53. doi: 10.1517/14712598.2010.495383. Expert Opin Biol Ther. 2010. PMID: 20515292 Review.
-
Effectiveness and safety of chemotherapy combined with cytokine-induced killer cell /dendritic cell-cytokine-induced killer cell therapy for treatment of gastric cancer in China: A systematic review and meta-analysis.Cytotherapy. 2016 Sep;18(9):1162-77. doi: 10.1016/j.jcyt.2016.05.015. Epub 2016 Jul 12. Cytotherapy. 2016. PMID: 27421742
Cited by
-
Oncolytic Viro-Immunotherapy: An Emerging Option in the Treatment of Gliomas.Front Immunol. 2021 Oct 5;12:721830. doi: 10.3389/fimmu.2021.721830. eCollection 2021. Front Immunol. 2021. PMID: 34675919 Free PMC article. Review.
-
Oncolytic Virus Combination Therapy: Killing One Bird with Two Stones.Mol Ther. 2018 Jun 6;26(6):1414-1422. doi: 10.1016/j.ymthe.2018.04.001. Epub 2018 Apr 5. Mol Ther. 2018. PMID: 29703699 Free PMC article. Review.
-
White paper on microbial anti-cancer therapy and prevention.J Immunother Cancer. 2018 Aug 6;6(1):78. doi: 10.1186/s40425-018-0381-3. J Immunother Cancer. 2018. PMID: 30081947 Free PMC article. Review.
-
Expression of Lymphocyte-Activation Gene 3 (LAG-3) Immune Checkpoint Receptor Identifies a Tumor-Reactive T Cell Population in the Peripheral Blood of Patients with Colorectal Cancer.Med Sci Monit. 2019 May 11;25:3495-3502. doi: 10.12659/MSM.915741. Med Sci Monit. 2019. Retraction in: Med Sci Monit. 2019 Jun 13;25:4400. doi: 10.12659/MSM.917907. PMID: 31077581 Free PMC article. Retracted.
-
Restored CD8+PD-1+ T Cells Facilitate the Response to Anti-PD-1 for Patients With Pancreatic Ductal Adenocarcinoma.Front Oncol. 2022 Apr 11;12:837560. doi: 10.3389/fonc.2022.837560. eCollection 2022. Front Oncol. 2022. PMID: 35480107 Free PMC article.
References
-
- Moehler M, Blechacz B, Weiskopf N, Zeidler M, Stremmel W, Rommelaere J, Galle PR, Cornelis JJ. Effective infection, apoptotic cell killing and gene transfer of human hepatoma cells but not primary hepatocytes by parvovirus H1 and derived vectors. Cancer Gene Ther 2001; 8:158-67; PMID:11332986; http://dx.doi.org/10.1038/sj.cgt.7700288 - DOI - PubMed
-
- Donnelly O, Harrington K, Melcher A, Pandha H. Live viruses to treat cancer. J R Soc Med 2013; 106:310-4; PMID:23824333; http://dx.doi.org/10.1177/0141076813494196 - DOI - PMC - PubMed
-
- Russell SJ, Peng KW, Bell JC. Oncolytic virotherapy. Nat Biotechnol 2012; 30:658-70; PMID:22781695; http://dx.doi.org/10.1038/nbt.2287 - DOI - PMC - PubMed
-
- Atherton MJ, Lichty BD. Evolution of oncolytic viruses: novel strategies for cancer treatment. Immunotherapy 2013; 5:1191-206; PMID:24188674; http://dx.doi.org/10.2217/imt.13.123 - DOI - PubMed
-
- Carpenter SG, Carson J, Fong Y. Regional liver therapy using oncolytic virus to target hepatic colorectal metastases. Semin Oncol 2010; 37:160-69; PMID:20494708; http://dx.doi.org/10.1053/j.seminoncol.2010.03.001 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
