Preclinical efficacy of immune-checkpoint monotherapy does not recapitulate corresponding biomarkers-based clinical predictions in glioblastoma
- PMID: 28507806
- PMCID: PMC5414887
- DOI: 10.1080/2162402X.2017.1295903
Preclinical efficacy of immune-checkpoint monotherapy does not recapitulate corresponding biomarkers-based clinical predictions in glioblastoma
Abstract
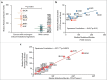
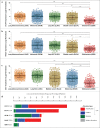
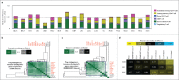
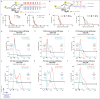
Glioblastoma (GBM) is resistant to most multimodal therapies. Clinical success of immune-checkpoint inhibitors (ICIs) has spurred interest in applying ICIs targeting CTLA4, PD1 or IDO1 against GBM. This amplifies the need to ascertain GBM's intrinsic susceptibility (or resistance) toward these ICIs, through clinical biomarkers that may also "guide and prioritize" preclinical testing. Here, we interrogated the TCGA and/or REMBRANDT human patient-cohorts to predict GBM's predisposition toward ICIs. We exploited various broad clinical biomarkers, including mutational or predicted-neoantigen burden, pre-existing or basal levels of tumor-infiltrating T lymphocytes (TILs), differential expression of immune-checkpoints within the tumor and their correlation with particular TILs/Treg-associated functional signature and prognostic impact of differential immune-checkpoint expression. Based on these analyses, we found that predictive biomarkers of ICI responsiveness exhibited inconsistent patterns in GBM patients, i.e., they either predicted ICI resistance (as compared with typical ICI-responsive cancer-types like melanoma, lung cancer or bladder cancer) or susceptibility to therapeutic targeting of CTLA4 or IDO1. On the other hand, our comprehensive literature meta-analysis and preclinical testing of ICIs using an orthotopic GL261-glioma mice model, indicated significant antitumor properties of anti-PD1 antibody, whereas blockade of IDO1 or CTLA4 either failed or provided very marginal advantage. These trends raise the need to better assess the applicability of ICIs and associated biomarkers for GBM.
Keywords: CTLA4; Cancer immunotherapy; IDO1; PD1; T cells; Treg cells; copy-number alterations (CNA); high-grade glioma; immune-checkpoint blockers (ICBs); mutational burden; neoantigen; patient prognosis.
Figures


Comment in
-
Commentary: preclinical efficacy of immune-checkpoint monotherapy does not recapitulate corresponding biomarkers-based clinical predictions in glioblastoma by Garg et al. (2017).Oncoimmunology. 2018 Dec 13;8(3):1548242. doi: 10.1080/2162402X.2018.1548242. eCollection 2019. Oncoimmunology. 2018. PMID: 30723577 Free PMC article.
References
-
- Johanns TM, Ward JP, Miller CA, Wilson C, Kobayashi DK, Bender D, Fu Y, Alexandrov A, Mardis ER, Artyomov MN et al.. Endogenous neoantigen-specific CD8 T cells identified in two glioblastoma models using a cancer immunogenomics approach. Cancer Immunol Res 2016; 4:1007-15; PMID:27799140; http://dx.doi.org/10.1158/2326-6066.CIR-16-0156 - DOI - PMC - PubMed
-
- Preusser M, Lim M, Hafler DA, Reardon DA, Sampson JH. Prospects of immune checkpoint modulators in the treatment of glioblastoma. Nat Rev Neurol 2015; 11:504-14; PMID:26260659; http://dx.doi.org/10.1038/nrneurol.2015.139 - DOI - PMC - PubMed
-
- Polyzoidis S, Tuazon J, Brazil L, Beaney R, Al-Sarraj ST, Doey L, Logan J, Hurwitz V, Jarosz J, Bhangoo R et al.. Active dendritic cell immunotherapy for glioblastoma: Current status and challenges. Br J Neurosurg 2015; 29:197-205; PMID:25541743; http://dx.doi.org/10.3109/02688697.2014.994473 - DOI - PubMed
-
- Anguille S, Smits EL, Lion E, van Tendeloo VF, Berneman ZN. Clinical use of dendritic cells for cancer therapy. Lancet Oncol 2014; 15:e257-67; PMID:24872109; http://dx.doi.org/10.1016/S1470-2045(13)70585-0 - DOI - PubMed
-
- Garg AD, Vandenberk L, Koks C, Verschuere T, Boon L, Van Gool SW, Agostinis P. Dendritic cell vaccines based on immunogenic cell death elicit danger signals and T cell-driven rejection of high-grade glioma. Sci Transl Med 2016; 8:328ra27; http://dx.doi.org/10.1126/scitranslmed.aae0105 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
