The Vitamin D for Enhancing the Immune System in Cystic Fibrosis (DISC) trial: Rationale and design of a multi-center, double-blind, placebo-controlled trial of high dose bolus administration of vitamin D3 during acute pulmonary exacerbation of cystic fibrosis
- PMID: 28508087
- PMCID: PMC5427007
- DOI: 10.1016/j.conctc.2017.02.010
The Vitamin D for Enhancing the Immune System in Cystic Fibrosis (DISC) trial: Rationale and design of a multi-center, double-blind, placebo-controlled trial of high dose bolus administration of vitamin D3 during acute pulmonary exacerbation of cystic fibrosis
Abstract
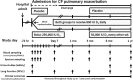
Vitamin D deficiency is highly prevalent in children and adults with cystic fibrosis (CF). Recent studies have found an association between vitamin D status and risk of pulmonary exacerbations in children and adults with CF. The ongoing Vitamin D for enhancing the Immune System in Cystic fibrosis (DISC) study is a multi-center, double-blind, randomized, placebo-controlled trial that will test the hypothesis of whether high dose vitamin D given as a single oral bolus of 250,000 IU to adults with CF during a pulmonary exacerbation followed by a maintenance dose of vitamin D will improve time to next pulmonary exacerbation and re-hospitalization, improve survival and lung function compared to placebo and reduce the rates of pulmonary exacerbation,. Subjects will be randomized 1:1 at each clinical site to vitamin D or placebo within 72 hours of hospital admission for pulmonary exacerbation. Clinical follow-up visits will occur at 1, 2, 3, and 7 days, and 1, 3, 6 and 12 months after randomization. Blood and sputum will be collected and determination of clinical outcomes will be assessed at each visit. The primary endpoint will be the time to next pulmonary exacerbation requiring antibiotics, re-hospitalization or death. The secondary endpoints will include lung function assessed by forced expiratory volume in 1 second (FEV1), blood markers of inflammatory cytokines, anti-microbial peptide expression by peripheral blood mononuclear cells and circulating concentrations in blood. Other exploratory endpoints will examine the phenotype of neutrophils and monocyte/macrophages in sputum. Nutritional status will be assessed by 3 day food records and food frequency questionnaire.
Keywords: Vitamin D; cystic fibrosis; diet; nutrition; pulmonary exacerbation; randomized controlled trial.
Figures
References
-
- Strausbaugh S.D., Davis P.B. Cystic fibrosis: a review of epidemiology and pathobiology. Clin. Chest Med. 2007;28(2):279–288. - PubMed
-
- Elborn J.S. Cystic fibrosis. Lancet. 2016 Apr 29 pii: S0140–6736(16)00576-6 [Epub ahead of print]
-
- Ode K.L., Moran A. New insights into cystic fibrosis-related diabetes in children. Lancet Diabetes Endocrinol. 2013 Sep;1(1):52–58. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources