Testosterone insulin-like effects: an in vitro study on the short-term metabolic effects of testosterone in human skeletal muscle cells
- PMID: 28508346
- PMCID: PMC5610223
- DOI: 10.1007/s40618-017-0686-y
Testosterone insulin-like effects: an in vitro study on the short-term metabolic effects of testosterone in human skeletal muscle cells
Abstract
Purpose: Testosterone by promoting different metabolic pathways contributes to short-term homeostasis of skeletal muscle, the largest insulin-sensitive tissue and the primary site for insulin-stimulated glucose utilization. Despite evidences indicate a close relationship between testosterone and glucose metabolism, the molecular mechanisms responsible for a possible testosterone-mediated insulin-like effects on skeletal muscle are still unknown.
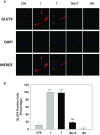
Methods: Here we used undifferentiated proliferating or differentiated human fetal skeletal muscle cells (Hfsmc) to investigate the short-term effects of testosterone on the insulin-mediated biomolecular metabolic machinery. GLUT4 cell expression, localization and the phosphorylation/activation of AKT, ERK, mTOR and GSK3β insulin-related pathways at different time points after treatment with testosterone were analyzed.
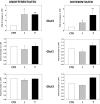
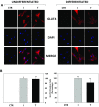
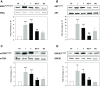
Results: Independently from cells differentiation status, testosterone, with an insulin-like effect, induced Glut4-mRNA expression, GLUT4 protein translocation to the cytoplasmic membrane, while no effect was observed on GLUT4 protein expression levels. Furthermore, testosterone treatment modulated the insulin-dependent signal transduction pathways inducing a rapid and persistent activation of AKT, ERK and mTOR, and a transient inhibition of GSK3β. T-related effects were shown to be androgen receptor dependent.
Conclusion: All together our data indicate that testosterone through the activation of non-genomic pathways, participates in skeletal muscle glucose metabolism by inducing insulin-related effects.
Keywords: Human skeletal muscle cells; Insulin; Metabolism; Testosterone.
Conflict of interest statement
Conflict of interest
The authors have nothing to declare and no conflict of interest. All authors have read and approved the final manuscript.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study formal consent is not required.
Funding
This work was funded by grants from the Italian Ministry of Instruction, University and Research (PRIN 2010C8ERKX_002).
Figures

Similar articles
-
Comparative study of testosterone and vitamin D analogue, elocalcitol, on insulin-controlled signal transduction pathway regulation in human skeletal muscle cells.J Endocrinol Invest. 2019 Aug;42(8):897-907. doi: 10.1007/s40618-018-0998-6. Epub 2019 Jan 1. J Endocrinol Invest. 2019. PMID: 30600434
-
Resistin inhibits glucose uptake in L6 cells independently of changes in insulin signaling and GLUT4 translocation.Am J Physiol Endocrinol Metab. 2003 Jul;285(1):E106-15. doi: 10.1152/ajpendo.00457.2002. Epub 2003 Mar 4. Am J Physiol Endocrinol Metab. 2003. PMID: 12618360
-
AMPK activation by prolonged stimulation with interleukin-1β contributes to the promotion of GLUT4 translocation in skeletal muscle cells.Cell Biol Int. 2016 Nov;40(11):1204-1211. doi: 10.1002/cbin.10673. Epub 2016 Sep 13. Cell Biol Int. 2016. PMID: 27569904
-
Time-dependent effects of fatty acids on skeletal muscle metabolism.J Cell Physiol. 2007 Jan;210(1):7-15. doi: 10.1002/jcp.20811. J Cell Physiol. 2007. PMID: 17013887 Review.
-
Insulin signaling: a potential signaling pathway for the stimulatory effect of kaempferitrin on glucose uptake in skeletal muscle.Eur J Pharmacol. 2013 Jul 15;712(1-3):1-7. doi: 10.1016/j.ejphar.2013.02.029. Epub 2013 Mar 1. Eur J Pharmacol. 2013. PMID: 23458067 Review.
Cited by
-
Vitamin D as a Shield against Aging.Int J Mol Sci. 2023 Feb 25;24(5):4546. doi: 10.3390/ijms24054546. Int J Mol Sci. 2023. PMID: 36901976 Free PMC article. Review.
-
Metabolic dysfunction in pulmonary hypertension: from basic science to clinical practice.Eur Respir Rev. 2017 Dec 20;26(146):170094. doi: 10.1183/16000617.0094-2017. Print 2017 Dec 31. Eur Respir Rev. 2017. PMID: 29263174 Free PMC article. Review.
-
Cardiorespiratory, enzymatic and hormonal responses during and after walking while fasting.PLoS One. 2018 Mar 1;13(3):e0193702. doi: 10.1371/journal.pone.0193702. eCollection 2018. PLoS One. 2018. PMID: 29494664 Free PMC article.
-
Androgen receptor regulates the proliferation of myoblasts under appropriate or excessive stretch through IGF-1 receptor mediated p38 and ERK1/2 pathways.Nutr Metab (Lond). 2021 Sep 15;18(1):85. doi: 10.1186/s12986-021-00610-y. Nutr Metab (Lond). 2021. PMID: 34526063 Free PMC article.
-
Change in Visceral Fat and Total Body Fat and the Effect on Cardiometabolic Risk Factors During Transgender Hormone Therapy.J Clin Endocrinol Metab. 2022 Jan 1;107(1):e153-e164. doi: 10.1210/clinem/dgab616. J Clin Endocrinol Metab. 2022. PMID: 34415999 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

