Serological weak D phenotypes: a review and guidance for interpreting the RhD blood type using the RHD genotype
- PMID: 28508413
- PMCID: PMC5612847
- DOI: 10.1111/bjh.14757
Serological weak D phenotypes: a review and guidance for interpreting the RhD blood type using the RHD genotype
Abstract
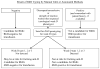
Approximately 0·2-1% of routine RhD blood typings result in a "serological weak D phenotype." For more than 50 years, serological weak D phenotypes have been managed by policies to protect RhD-negative women of child-bearing potential from exposure to weak D antigens. Typically, blood donors with a serological weak D phenotype have been managed as RhD-positive, in contrast to transfusion recipients and pregnant women, who have been managed as RhD-negative. Most serological weak D phenotypes in Caucasians express molecularly defined weak D types 1, 2 or 3 and can be managed safely as RhD-positive, eliminating unnecessary injections of Rh immune globulin and conserving limited supplies of RhD-negative RBCs. If laboratories in the UK, Ireland and other European countries validated the use of potent anti-D reagents to result in weak D types 1, 2 and 3 typing initially as RhD-positive, such laboratory results would not require further testing. When serological weak D phenotypes are detected, laboratories should complete RhD testing by determining RHD genotypes (internally or by referral). Individuals with a serological weak D phenotype should be managed as RhD-positive or RhD-negative, according to their RHD genotype.
Keywords: RHD gene; RhD blood group; blood transfusion; partial D phenotype; serological weak D phenotype.
© 2017 John Wiley & Sons Ltd.
Conflict of interest statement
Figures
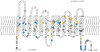
References
-
- Agre PC, Davies DM, Issitt PD, Lamy BM, Schmidt PJ, Treacy M, Vengelen-Tyler V. A proposal to standardize terminology for weak D antigen. Transfusion. 1992;32:86–7. - PubMed
-
- Argall CI, Ball JM, Trentelman E. Presence of anti-D antibody in the serum of a DU patient. Journal of Laboratory and Clinical Medicine. 1953;41:895–898. - PubMed
-
- Cannon M, Pierce R, Taber EB, Schucker J. Fatal hydrops fetalis caused by anti-D in a mother with partial D. Obstetrics and Gynecology. 2003;102:1143–5. - PubMed
-
- Chou ST, Jackson T, Vege S, Smith-Whitley K, Friedman DF, Westhoff CM. High prevalence of red blood cell alloimmunization in sickle cell disease despite transfusion from Rh-matched minority donors. Blood. 2013;122:1062–1071. - PubMed
-
- Contreras M, Knight RC. The Rh-negative donor. Clinical Laboratory Haematology. 1989;11:317–322. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

