Macrophages are necessary for epimorphic regeneration in African spiny mice
- PMID: 28508748
- PMCID: PMC5433844
- DOI: 10.7554/eLife.24623
Macrophages are necessary for epimorphic regeneration in African spiny mice
Abstract
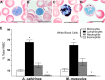
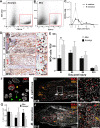
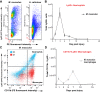
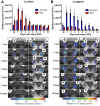
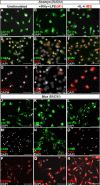
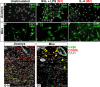
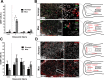
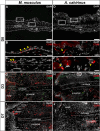
How the immune system affects tissue regeneration is not well understood. In this study, we used an emerging mammalian model of epimorphic regeneration, the African spiny mouse, to examine cell-based inflammation and tested the hypothesis that macrophages are necessary for regeneration. By directly comparing inflammatory cell activation in a 4 mm ear injury during regeneration (Acomys cahirinus) and scarring (Mus musculus), we found that both species exhibited an acute inflammatory response, with scarring characterized by stronger myeloperoxidase activity. In contrast, ROS production was stronger and more persistent during regeneration. By depleting macrophages during injury, we demonstrate a functional requirement for these cells to stimulate regeneration. Importantly, the spatial distribution of activated macrophage subtypes was unique during regeneration with pro-inflammatory macrophages failing to infiltrate the regeneration blastema. Together, our results demonstrate an essential role for inflammatory cells to regulate a regenerative response.
Keywords: Acomys; cell biology; developmental biology; epimorphic regeneration; inflammation; macrophages; mouse; stem cells.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures



Similar articles
-
Comparative analysis of ear-hole closure identifies epimorphic regeneration as a discrete trait in mammals.Nat Commun. 2016 Apr 25;7:11164. doi: 10.1038/ncomms11164. Nat Commun. 2016. PMID: 27109826 Free PMC article.
-
Tissue-resident macrophages specifically express Lactotransferrin and Vegfc during ear pinna regeneration in spiny mice.Dev Cell. 2024 Feb 26;59(4):496-516.e6. doi: 10.1016/j.devcel.2023.12.017. Epub 2024 Jan 15. Dev Cell. 2024. PMID: 38228141 Free PMC article.
-
Optimal skin regeneration after full thickness thermal burn injury in the spiny mouse, Acomys cahirinus.Burns. 2018 Sep;44(6):1509-1520. doi: 10.1016/j.burns.2018.05.018. Epub 2018 Jun 11. Burns. 2018. PMID: 29903601
-
Model systems for regeneration: the spiny mouse, Acomys cahirinus.Development. 2020 Feb 25;147(4):dev167718. doi: 10.1242/dev.167718. Development. 2020. PMID: 32098790 Free PMC article. Review.
-
Insights into the regeneration of skin from Acomys, the spiny mouse.Exp Dermatol. 2019 Apr;28(4):436-441. doi: 10.1111/exd.13847. Epub 2019 Jan 15. Exp Dermatol. 2019. PMID: 30457673 Review.
Cited by
-
Integrative biology of injury in animals.Biol Rev Camb Philos Soc. 2023 Feb;98(1):34-62. doi: 10.1111/brv.12894. Epub 2022 Sep 29. Biol Rev Camb Philos Soc. 2023. PMID: 36176189 Free PMC article. Review.
-
Opioids prevent regeneration in adult mammals through inhibition of ROS production.Sci Rep. 2018 Aug 15;8(1):12170. doi: 10.1038/s41598-018-29594-1. Sci Rep. 2018. PMID: 30111876 Free PMC article.
-
Comparative Proteomic Analysis in Scar-Free Skin Regeneration in Acomys cahirinus and Scarring Mus musculus.Sci Rep. 2020 Jan 13;10(1):166. doi: 10.1038/s41598-019-56823-y. Sci Rep. 2020. PMID: 31932597 Free PMC article.
-
Myc beyond Cancer: Regulation of Mammalian Tissue Regeneration.Pathophysiology. 2023 Aug 2;30(3):346-365. doi: 10.3390/pathophysiology30030027. Pathophysiology. 2023. PMID: 37606389 Free PMC article. Review.
-
Spiny mice activate unique transcriptional programs after severe kidney injury regenerating organ function without fibrosis.iScience. 2021 Nov 3;24(11):103269. doi: 10.1016/j.isci.2021.103269. eCollection 2021 Nov 19. iScience. 2021. PMID: 34849462 Free PMC article.
References
-
- Alexander KA, Chang MK, Maylin ER, Kohler T, Müller R, Wu AC, Van Rooijen N, Sweet MJ, Hume DA, Raggatt LJ, Pettit AR. Osteal macrophages promote in vivo intramembranous bone healing in a mouse tibial injury model. Journal of Bone and Mineral Research. 2011;26:1517–1532. doi: 10.1002/jbmr.354. - DOI - PubMed
-
- Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, Gherardi RK, Chazaud B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. The Journal of Experimental Medicine. 2007;204:1057–1069. doi: 10.1084/jem.20070075. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

