Untargeted metabolomic analysis of tomato pollen development and heat stress response
- PMID: 28508929
- PMCID: PMC5486769
- DOI: 10.1007/s00497-017-0301-6
Untargeted metabolomic analysis of tomato pollen development and heat stress response
Abstract
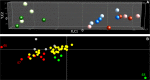
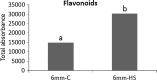
Pollen development metabolomics. Developing pollen is among the plant structures most sensitive to high temperatures, and a decrease in pollen viability is often associated with an alteration of metabolite content. Most of the metabolic studies of pollen have focused on a specific group of compounds, which limits the identification of physiologically important metabolites. To get a better insight into pollen development and the pollen heat stress response, we used a liquid chromatography-mass spectrometry platform to detect secondary metabolites in pollen of tomato (Solanum lycopersicum L.) at three developmental stages under control conditions and after a short heat stress at 38 °C. Under control conditions, the young microspores accumulated a large amount of alkaloids and polyamines, whereas the mature pollen strongly accumulated flavonoids. The heat stress treatment led to accumulation of flavonoids in the microspore. The biological role of the detected metabolites is discussed. This study provides the first untargeted metabolomic analysis of developing pollen under a changing environment that can serve as reference for further studies.
Keywords: Heat stress; High temperature; Metabolomics; Pollen development; Untargeted analysis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Altman A, Levin N. Interactions of polyamines and nitrogen nutrition in plants. Physiol Plant. 1993;89:653–658. doi: 10.1111/j.1399-3054.1993.tb05229.x. - DOI
-
- Bagni N, Scaramagli S, Bueno M, Della Mea M, Torrigiani P (1994) Which is the active form of polyamines in plants? In: Caldarera CM, Clò C, Moruzzi MS (eds) Polyamines: biological and clinical aspects. CLUEB, Bologna, pp 131–137
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

