Successful treatment with tolvaptan to control blood volume and hyponatremia in a chronic kidney disease patient
- PMID: 28509063
- PMCID: PMC5411527
- DOI: 10.1007/s13730-012-0018-1
Successful treatment with tolvaptan to control blood volume and hyponatremia in a chronic kidney disease patient
Abstract
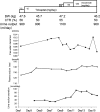
We report a case of successful treatment with tolvaptan (15 mg/day) in a 73-year-old female patient with chronic kidney disease (CKD) stage 5 due to diabetic nephropathy and renal sclerosis for volume control and loop diuretic-induced hyponatremia. Her creatinine clearance has remained at 7-10 ml/min for the last 6 months. She was treated by dietary and drug therapy, namely, antihypertensives (nifedipine: 40 mg/day, olmesartan: 20 mg/day) and loop diuretics (azosemide: 40-120 mg/day), for CKD and concomitant diseases of hypertension and diabetic mellitus. She developed loop diuretic-induced hyponatremia (120 mmol/l) by increased sodium excretion, but the diuretic was required for the control of volume overload. Hence, azosemide was suspended and tolvaptan (15 mg/day) was administered. After tolvaptan treatment, the plasma sodium level gradually increased to a normal level (135-140 mmol/l) and volume overload was improved. Urine volume was maintained at about 1000 ml/day with low sodium excretion (<40 mmol/day) and increased free water clearance. These results suggest that tolvaptan may be effective for volume control and diuretic-induced hyponatremia in CKD patients.
Keywords: Chronic kidney disease; Hyponatremia; Tolvaptan.
Figures
References
-
- Buckalew VM, Jr, Berg RL, Wang SR, Porush JG, Rauch S, Schulman G. Prevalence of hypertension in 1,795 subjects with chronic renal disease: the modification of diet in renal disease study baseline cohort. Modification of Diet in Renal Disease Study Group. Am J Kidney Dis. 1996;28(6):811–821. doi: 10.1016/S0272-6386(96)90380-7. - DOI - PubMed
LinkOut - more resources
Full Text Sources