The excitation-contraction coupling mechanism in skeletal muscle
- PMID: 28509964
- PMCID: PMC5425715
- DOI: 10.1007/s12551-013-0135-x
The excitation-contraction coupling mechanism in skeletal muscle
Abstract
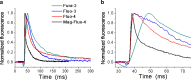
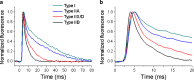
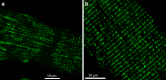
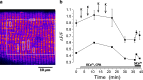
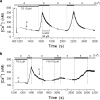
First coined by Alexander Sandow in 1952, the term excitation-contraction coupling (ECC) describes the rapid communication between electrical events occurring in the plasma membrane of skeletal muscle fibres and Ca2+ release from the SR, which leads to contraction. The sequence of events in twitch skeletal muscle involves: (1) initiation and propagation of an action potential along the plasma membrane, (2) spread of the potential throughout the transverse tubule system (T-tubule system), (3) dihydropyridine receptors (DHPR)-mediated detection of changes in membrane potential, (4) allosteric interaction between DHPR and sarcoplasmic reticulum (SR) ryanodine receptors (RyR), (5) release of Ca2+ from the SR and transient increase of Ca2+ concentration in the myoplasm, (6) activation of the myoplasmic Ca2+ buffering system and the contractile apparatus, followed by (7) Ca2+ disappearance from the myoplasm mediated mainly by its reuptake by the SR through the SR Ca2+ adenosine triphosphatase (SERCA), and under several conditions movement to the mitochondria and extrusion by the Na+/Ca2+ exchanger (NCX). In this text, we review the basics of ECC in skeletal muscle and the techniques used to study it. Moreover, we highlight some recent advances and point out gaps in knowledge on particular issues related to ECC such as (1) DHPR-RyR molecular interaction, (2) differences regarding fibre types, (3) its alteration during muscle fatigue, (4) the role of mitochondria and store-operated Ca2+ entry in the general ECC sequence, (5) contractile potentiators, and (6) Ca2+ sparks.
Keywords: Ca2+ transients; Excitation–contraction coupling; Fibre types; Mitochondria; Skeletal muscle.
Figures

References
-
- Abbiss C, Laursen P. Models to explain fatigue during prolonged endurance cycling. Sports Med. 2005;35:865–898. - PubMed
-
- Adams B, Tanabe T, Mikami A, Numa S, Beam K. Intramembrane charge movement restored in dysgenic skeletal muscle by injection of dihydropyridine receptor cDNAs. Nature. 1990;346:569–572. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

