Proteoliposomes in nanobiotechnology
- PMID: 28510001
- PMCID: PMC5418368
- DOI: 10.1007/s12551-011-0065-4
Proteoliposomes in nanobiotechnology
Abstract
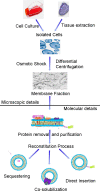
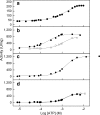
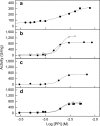
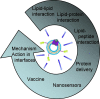
Proteoliposomes are systems that mimic lipid membranes (liposomes) to which a protein has been incorporated or inserted. During the last decade, these systems have gained prominence as tools for biophysical studies on lipid-protein interactions as well as for their biotechnological applications. Proteoliposomes have a major advantage when compared with natural membrane systems, since they can be obtained with a smaller number of lipidic (and protein) components, facilitating the design and interpretation of certain experiments. However, they have the disadvantage of requiring methodological standardization for incorporation of each specific protein, and the need to verify that the reconstitution procedure has yielded the correct orientation of the protein in the proteoliposome system with recovery of its functional activity. In this review, we chose two proteins under study in our laboratory to exemplify the steps necessary for the standardization of the reconstitution of membrane proteins in liposome systems: (1) alkaline phosphatase, a protein with a glycosylphosphatidylinositol anchor, and (2) Na,K-ATPase, an integral membrane protein. In these examples, we focus on the production of the specific proteoliposomes, as well as on their biochemical and biophysical characterization, with emphasis on studies of lipid-protein interactions. We conclude the chapter by highlighting current prospects of this technology for biotechnological applications, including the construction of nanosensors and of a multi-protein nanovesicular biomimetic to study the processes of initiation of skeletal mineralization.
Keywords: Alkaline phosphatase; Biomineralization; Biotechnology; Leishmaniasis; Na,K-ATPase; Nanosensor; Proteoliposomes.
Figures
References
-
- Albers RW. Biochemical aspects of active transport. Ann Biochem. 1967;36:727–756. - PubMed
-
- Alves JB, Ferreira CL, Moreira AF, Silva GA, Alves GD, Paulino TP, Ciancaglini P, Thedei G, Jr, Napimoga MH. Local delivery of EGF-liposome mediated bone modeling in orthodontic tooth movement by increasing RANKL expression. Life Sci. 2009;85(19–20):693–699. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

