Dissociative mechanism for irreversible thermal denaturation of oligomeric proteins
- PMID: 28510015
- PMCID: PMC5418479
- DOI: 10.1007/s12551-016-0220-z
Dissociative mechanism for irreversible thermal denaturation of oligomeric proteins
Abstract
Protein stability is a fundamental characteristic essential for understanding conformational transformations of the proteins in the cell. When using protein preparations in biotechnology and biomedicine, the problem of protein stability is of great importance. The kinetics of denaturation of oligomeric proteins may have characteristic properties determined by the quaternary structure. The kinetic schemes of denaturation can include the multiple stages of conformational transitions in the protein oligomer and stages of reversible dissociation of the oligomer. In this case, the shape of the kinetic curve of denaturation or the shape of the melting curve registered by differential scanning calorimetry can vary with varying the protein concentration. The experimental data illustrating dissociative mechanism for irreversible thermal denaturation of oligomeric proteins have been summarized in the present review. The use of test systems based on thermal aggregation of oligomeric proteins for screening of agents possessing anti-aggregation activity is discussed.
Keywords: Conformational lock; Dissociative mechanism; Oligomeric proteins; Protein denaturation.
Conflict of interest statement
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Figures
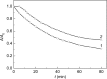
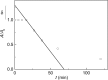



References
-
- Amani M, Moosavi-Movahedi AA, Floris G, Longu S, Mura A, Moosavi-Nejad SZ, Saboury AA, Ahmad F. Comparative study of the conformational lock, dissociative thermal inactivation and stability of euphorbia latex and lentil seedling amine oxidases. Protein J. 2005;24:183–191. doi: 10.1007/s10930-005-7842-5. - DOI - PubMed
-
- Atyaksheva LF, Pilipenko OS, Chukhrai ES, Poltorak OM. Similarity of and differences between the mechanisms of thermal inactivation of β-galactosidases of different origins. Russ J Phys Chem A. 2008;82:864–869. doi: 10.1134/S0036024408050300. - DOI
-
- Atyaksheva LF, Tarasevich BN, Chukhrai ES, Poltorak OM. Thermal inactivation of alkali phosphatases under various conditions. Russ J Phys Chem A. 2009;83:318–323. doi: 10.1134/S0036024409020307. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

