Contractility assessment in enzymatically isolated cardiomyocytes
- PMID: 28510074
- PMCID: PMC5425706
- DOI: 10.1007/s12551-012-0082-y
Contractility assessment in enzymatically isolated cardiomyocytes
Abstract
The use of enzymatically isolated cardiac myocytes is ubiquitous in modern cardiovascular research. Parallels established between cardiomyocyte shortening responses and those of intact tissue make the cardiomyocyte an invaluable experimental model of cardiac function. Much of our understanding regarding the fundamental processes underlying heart function is owed to our increasing capabilities in single-cell stimulation and direct or indirect observation, as well as quantitative analysis of such cells. Of the many important mechanisms and functions that can be readily assessed in cardiomyocytes at all stages of development, contractility is the most representative and one of the most revealing. The purpose of this review is to provide a survey of various methodological approaches in the literature used to assess adult and neonatal cardiomyocyte contractility. The various methods employed to evaluate the contractile behavior of enzymatically isolated mammalian cardiac myocytes can be conveniently divided into two general categories-those employing optical (image)-based systems and those that use transducer-based technologies. This survey is by no means complete, but we have made an effort to include the most popular methods in terms of reliability and accessibility. These techniques are in constant evolution and hold great promise for the next generation of breakthrough studies in cell biology for the prevention, treatment, and cure of cardiovascular diseases.
Keywords: Cardiac myocyte; Cardiomyocyte; Cardiovascular research; Contractility; Heart contraction; Sarcomere length.
Figures
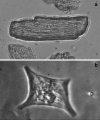



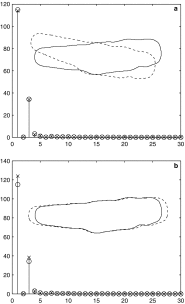
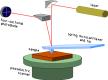
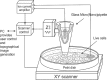
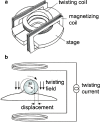
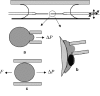

References
Publication types
LinkOut - more resources
Full Text Sources

