Conformational flexibility of N-glycans in solution studied by REMD simulations
- PMID: 28510079
- PMCID: PMC5418406
- DOI: 10.1007/s12551-012-0090-y
Conformational flexibility of N-glycans in solution studied by REMD simulations
Abstract
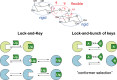
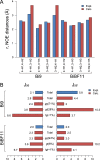
Protein-glycan recognition regulates a wide range of biological and pathogenic processes. Conformational diversity of glycans in solution is apparently incompatible with specific binding to their receptor proteins. One possibility is that among the different conformational states of a glycan, only one conformer is utilized for specific binding to a protein. However, the labile nature of glycans makes characterizing their conformational states a challenging issue. All-atom molecular dynamics (MD) simulations provide the atomic details of glycan structures in solution, but fairly extensive sampling is required for simulating the transitions between rotameric states. This difficulty limits application of conventional MD simulations to small fragments like di- and tri-saccharides. Replica-exchange molecular dynamics (REMD) simulation, with extensive sampling of structures in solution, provides a valuable way to identify a family of glycan conformers. This article reviews recent REMD simulations of glycans carried out by us or other research groups and provides new insights into the conformational equilibria of N-glycans and their alteration by chemical modification. We also emphasize the importance of statistical averaging over the multiple conformers of glycans for comparing simulation results with experimental observables. The results support the concept of "conformer selection" in protein-glycan recognition.
Keywords: Conformational flexibility; Conformer selection; Molecular dynamics simulations; N-glycan; N-glycan modifications; Protein–glycan interactions; Replica-exchange molecular dynamics simulations.
Figures


References
-
- André S, Unverzagt C, Kojima S, Frank M, Seifert J, Fink C, Kayser K, von der Lieth CW, Gabius HJ. Determination of modulation of ligand properties of synthetic complex-type biantennary N-glycans by introduction of bisecting GlcNAc in silico, in vitro and in vivo. Eur J Biochem. 2004;271(1):118–134. doi: 10.1046/j.1432-1033.2003.03910.x. - DOI - PubMed
-
- Beckham GT, Bomble YJ, Matthews JF, Taylor CB, Resch MG, Yarbrough JM, Decker SR, Bu LT, Zhao XC, McCabe C, Wohlert J, Bergenstråhle M, Brady JW, Adney WS, Himmel ME, Crowley MF. The O-glycosylated linker from the trichoderma reesei family 7 cellulase is a flexible, disordered protein. Biophys J. 2010;99(11):3773–3781. doi: 10.1016/j.bpj.2010.10.032. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

